Nicotine ranks among the oldest known plant alkaloids, first isolated from tobacco in the 1820s by chemists like Posselt and Reimann. The focus on tweaking the basic compound, especially to create salts like Nicotine Dihydrogen Ditartrate, picked up speed alongside advances in organic synthesis during the late nineteenth and early twentieth centuries. Pharmacologists were quick to notice that nicotine salts could open up new avenues for delivery and research. The shift from using plant extracts to working with well-defined salts reflected a broader drive to pin down consistent, measurable effects for research, industrial, and sometimes even medical use. The unfolding story of nicotine and its derivatives highlights a longstanding curiosity—how a chemical with such a checkered history might still deliver insights or practical value when steered by careful hands.
Nicotine Dihydrogen Ditartrate stands apart from regular nicotine through its stability and handling profile. Salts like this one draw in researchers and industry players because they dissolve more easily in water, offer reliable purity, and cut down on the volatility and irritancy tied to free-base nicotine. Chemists and formulators tend to reach for this compound where a measured, less harsh nicotine dose is needed. In the lab, this salt helps simplify dosing, blending, and exposure analysis compared to more reactive or oily nicotine forms. Commercial sources supply it as a white to off-white crystalline powder, fit for both bench-scale experiments and process-level work.
Under ordinary room temperatures, Nicotine Dihydrogen Ditartrate keeps to a solid, manageable form. Unlike oily liquid nicotine, this salt resists quick evaporation and stands up to brief temperature changes, which makes it less hazardous and easier to store outside a climate-controlled cabinet. Its water solubility stands as perhaps its most useful trait—lab workers can whip up precise solutions fast, supporting both analytical and synthesis work. Chemically, the molecule marries a nicotine base with tartaric acid, creating a dibasic salt. This pairing not only tames the strong nicotine odor but also buffers pH, offering predictability in reaction vessels or biological studies. Melt points for this compound typically land well above 150°C, another nod to its stability and shelf life under standard use.
Every bottle or pouch of Nicotine Dihydrogen Ditartrate needs a detailed label to clear regulatory and safety hurdles. Any responsible supplier details content by percent purity (usually exceeding 98% for research use), along with the lot or batch number, manufacture date, and recommended storage guidelines. The labeling spells out known hazards, following global standards. In practice, bulk users check not just on chemical specs, but also on physical grain or powder characteristics since flow and clumping can affect mixing or transfer in the lab. Typical specifications include moisture content limits, absence of heavy metals, and clarity about residual solvents—critical details for anyone chasing reproducible results, whether in a university or a commercial pilot plant.
Crafting Nicotine Dihydrogen Ditartrate tends to follow a well-trodden path. The process usually starts with purified, free-base nicotine, drawn directly from tobacco leaves or bioengineered sources. Chemists then react this base with tartaric acid (generally using a modest excess, fully dissolved in distilled water). The mixture is stirred, cooled, and monitored for pH until a solid begins to separate. After crystallization, filtration and thorough washing remove impurities and unreacted acid. The drying and sieving steps produce a powder, ready for immediate use or further quality checks. Some labs may use additional recrystallization steps if extreme purity is essential or if they need to meet tight pharma or regulatory standards.
Nicotine Dihydrogen Ditartrate sits at an interesting junction for chemical work. Researchers treat it as a staging post—serving both as a safe vehicle for pure nicotine and as a workable intermediate for synthesizing other salts, derivatives, or labeled analogs. The salt form softens some of the hazards that would otherwise mark alkylation, oxidation, or deuteration reactions. Many labs use this compound as a reference for dissolution studies, or emulsification for patch, spray, or gum formulations. On the modification side, chemists can adjust ratios or conditions to uncover mono-tartrate salts or blend in isotopic tracers, depending on downstream application. These patterns ripple out into pharmaceutical, agricultural, or bioscience studies, reflecting the utility of well-managed salt forms over free, unstable bases.
In research catalogs, technical bulletins, and procurement sheets, you’ll see Nicotine Dihydrogen Ditartrate listed under a tangle of names. Common entries include Nicotine Tartrate, Nicotine Hydrogen Tartrate, or (S)-2,3-Dihydroxybutanedioic acid; 3-[(2S)-1-Methylpyrrolidin-2-yl]pyridine salt. Some outlets slide a code number into their documents for tracking. When buying from global vendors, it helps to match on CAS number and molecular formula, since local labeling customs or translation can muddy the waters. No matter the name, trained buyers and handlers look for certificate of analysis documents and clear links to regulatory compliance paperwork.
Handling any nicotine salt, including this one, means working within a tight envelope of safety protocols. Even as a solid, the compound can poison through inhalation, ingestion, or skin contact. The move to salt form isn’t some magic shield against toxicity, so labs mandate gloves, eye protection, and well-ventilated spaces. Labels echo these warnings, flagging nicotine’s dangers for both acute and chronic health. Most chemical plants and university facilities keep Nicotine Dihydrogen Ditartrate locked up, tracking its use with logs and sometimes requiring supervisor sign-off for withdrawal. Disposal follows local hazardous waste rules, whether for spent solutions or process residues. Safety sheets emphasize rapid response to spills, need for approved fume hoods, and yet another critical item—regular staff training refreshers, even for experienced chemists.
Nicotine Dihydrogen Ditartrate carves out a niche in fields beyond tobacco research or manufacturing. In drug discovery labs, it helps characterize receptor response, map metabolism, or simulate nicotine dosing for new delivery systems. Biologists use it to probe brain circuits or test concepts around addiction, leveraging the salt’s solubility and stability. Companies chasing new nicotine replacement therapies lean on it for early formulation and comparison benchmarks. Analytical chemists pick this salt when calibrating methods for nicotine measurement in e-liquids, agricultural products, or biological samples. Its predictable handling means less error and less risk when comparing between studies—a rare plus in any work with an alkaloid as finicky as nicotine.
The R&D side of Nicotine Dihydrogen Ditartrate pulses with ongoing questions. Labs explore how the salt’s properties affect absorption, cellular response, or even mask bitterness for new pharma applications. Collaborative ventures between universities and industry groups measure its potential as a reference standard or as a delivery agent for non-tobacco nicotine. Scientists publish on its utility for controlled in vivo dosing, which bridges gaps between crude old-school extracts and unworkable pure nicotine. As more countries regulate or tax nicotine products, developers weigh how salt forms can fit new legal or health frameworks. The buzz around synthetic nicotine—untied to tobacco crops—hints at a wave of new production plants built around salts like this one, which already show their worth in established, well-cited labs.
If any point emerges clearly from current research, it’s this: salt forms do not erase nicotine’s intrinsic hazards. Toxicologists have mapped acute and chronic dose-response curves for decades, and while the salt base can slightly buffer harshness or volatility, it changes little at the receptor or systemic level. Studies still point to dangerous rapid absorption, high addiction risk, and broad-ranging toxic effects in mammals. Risk scientists use the salt to model workplace exposure, potential contact injuries, and environmental questions for disposal. The details feed into updated safety sheets, handling guides, and first-aid protocols that reflect real chemical data—not just old habits or folklore.
Looking forward, the role for Nicotine Dihydrogen Ditartrate likely hinges on expanding interest in nicotine science outside cigarettes or vaping. As public health debates sharpen, and the range of products widens, this salt will crop up in method validation, regulatory submissions, and side-by-side studies of relative harm or pharmacokinetics. If synthetic bio-manufacture overtakes leaf extraction, reproducible salts like this will play an even bigger role in manufacturing chains, with better batch tracking and fewer natural impurities. Digital tracking and more advanced hazard mitigation gear will keep the research pipeline open while blunting risks around human handling or accidental overexposure. The demand for clarity, traceability, and top-tier safety will only grow—pressuring labs and suppliers to push beyond the old ways and place bets on well-characterized intermediates like Nicotine Dihydrogen Ditartrate.
Nicotine has always brought a flood of controversy, especially as science draws a sharper picture of its health risks. But the discussion gains a different angle when talking about nicotine dihydrogen ditartrate. This compound doesn’t pop up much in news headlines, yet it quietly plays a role in the world of medicine, drug development, and research.
This specific form of nicotine isn’t something you’d find in a regular pack of cigarettes. It’s water-soluble and much more stable, making it useful in pharmaceutical labs. Formulators work with this compound when developing smoking cessation therapies. As people try to quit smoking, switching to a medication with controlled doses of nicotine can ease withdrawal. There are various nicotine replacement therapies (NRTs) —think gums, patches, lozenges—designed to give the body nicotine without inhaling smoke. Nicotine dihydrogen ditartrate often goes into these products. Its consistency helps ensure each tablet or lozenge delivers the right amount.
Research institutions and universities use this chemical in studies about nicotine addiction, cravings, and how the body reacts to different doses. Researchers prefer this salt because it dissolves reliably in water, making it easy to control experiments. For instance, experiments tracking how people react to nicotine can’t afford the messiness of working with pure nicotine oil. Nicotine dihydrogen ditartrate steps up as an easy-to-handle alternative. In my college research, we always chose salts like these when we needed to be precise. Sloppy data from poor control over dosing can ruin an entire project.
Animal health isn’t immune to nicotine’s effects. Certain formulations for pests or parasites in agriculture or animal care sometimes use diluted forms of this compound. Old literature points to its role in treating livestock infestation. These days, the market leans on safer and more effective options, but the historical precedent exists. If you look into older veterinary formularies, you find references to the tartrate salt because it balanced potency with shelf-life.
Healthcare demand keeps shifting. Vaping, alternative nicotine products, and changing legal environments spark new studies and therapies. Patents from pharmaceutical companies mention this compound as a core ingredient in measured dosing devices, especially when trying to reduce the risk of misuse. In a world where regulation tightens and patient safety comes to the forefront, reliable chemical forms like the tartrate become more valuable.
Of course, a compound rooted in nicotine isn’t free from scrutiny. There are questions about addiction, long-term safety, and the risk of teen use, even in medicinal products. I worked at a pharmacy for a few years and saw firsthand how supply chain oversight and careful labeling matter. Product misuse almost always stems from gaps in education and clear communication. I believe the industry benefits from stronger tracking systems and tighter packaging controls. Education—especially for younger or at-risk populations—should never fall to the back burner.
Nicotine dihydrogen ditartrate won’t be front-page news. But those working in healthcare, research, and regulation understand its practical value. As more solutions for nicotine addiction shape up, keeping eyes on ingredients—and how they affect the real world—remains key.
Nicotine comes loaded with baggage. Decades of cigarettes, addiction, disease, big lawsuits—all painted the word “nicotine” with warning labels in most people’s minds. Still, the chemical keeps finding new forms. Nicotine dihydrogen ditartrate landed on the market as another approach, usually in pharmaceutical or research settings. Some vape products and nicotine replacement therapies started using salt forms of nicotine, so questions about safety feel both real and urgent.
Nicotine dihydrogen ditartrate stands out because it’s a salt, not a freebase. This actually changes the way the compound dissolves in water and gets absorbed by the body. Pharmaceutical companies like it since it brings both solubility and a consistent dose. You’ll mostly find it in specialized research, and sometimes as a part of replacement products, not on every store shelf.
Nicotine carries real risks, regardless of what form it takes. Heart rate climbs, blood pressure follows, and people report headaches, dizziness, even nausea at higher doses. Long-term users don’t escape damage—nicotine drives addiction, increases the chance of atherosclerosis, and damages arteries. Salt forms can behave slightly differently in the body, but the core risks don’t disappear. No salt form of nicotine makes the compound a safe daily companion.
You can’t judge a drug just by one chemical tweak. I’ve talked with pharmacists who stress that any nicotine, whether from a patch, gum, or new compound, needs strict controls. Most regulatory agencies treat these newer salt forms with the same scrutiny as standard nicotine. FDA approvals for medicinal use take years of safety trials. These trials need to include every variation, since even small chemical differences can change how the drug works or causes harm.
There isn’t enough long-term, independent research showing that nicotine dihydrogen ditartrate is much “safer” than other forms. Basic side effects—nausea, fast heartbeat, sleep problems—don’t change because the body breaks down all nicotine to the same end products. Even minor chemical tweaks won’t help someone if the main ingredient itself causes harm.
Better oversight would help. Many products that use newer nicotine salts don’t provide clear dose labeling. That matters, especially since nicotine’s effects change quickly at different concentrations. If you’re looking for help quitting smoking, certified nicotine replacement therapies still serve as a safer first step, compared to jumping on trends promising “improved” or “safer” nicotine.
Schools and clinics need stronger education about nicotine in all its forms. One of my relatives picked up vaping to quit smoking, believing a “modern” nicotine salt meant zero risk. They ended up more addicted, buying stronger cartridges each week. The lesson: knowing the science makes a difference. Honest warnings and updated labels support real choice.
Pharmacies and health shops should only offer nicotine dihydrogen ditartrate under strict medical guidance. Studies suggest the best outcomes come with doctor supervision, supporting people who want to quit without confusing or misleading claims. Quality testing and clear instructions should back every product.
Safe doesn’t just mean “not cancer-causing.” Until research shows otherwise, nicotine in all forms brings serious risks. Salt or not, trust is earned with open data, clear guidance from experts, and responsible product development.
Nicotine dihydrogen ditartrate enters conversations most often among scientists, healthcare providers, and a few DIY e-liquid enthusiasts. It’s tough to spot it on pharmacy shelves, but in research and specialty settings, this salt of nicotine proves valuable for its solubility in water and stable composition. The nicotine part of the compound brings the same punch found in tobacco and vaping products.
Talk about nicotine, and the idea of dose means everything. Overshooting what the body tolerates leads to side effects—nausea, dizziness, or worse. Underdosing, and the pharmacological effect fails to materialize. The same holds doubly true for a salt like nicotine dihydrogen ditartrate, which doesn’t show up with an easy-to-read dosing chart in your medicine cabinet.
Researchers and compounding pharmacists call for exact math when measuring doses because nicotine salts have different weights compared to the classic nicotine base or the nicotine in cigarettes. 1 mg of pure nicotine does not match 1 mg of the tartrate salt. Instead, you need to calculate how much actual nicotine is present in the molecule—many rely on conversion factors. For example, only about 39% of the salt’s weight is pure nicotine.
Pharmaceutical studies and harm reduction projects sometimes refer to nicotine doses in milligrams per day, with transdermal patches, lozenges, and e-liquids all coming in different strengths. Clinical data often starts at about 1-2 mg of nicotine (not salt) per dose for nicotine replacement. For perspective, a single cigarette delivers about 1 to 2 mg of nicotine into the bloodstream; a pack per day habit means somewhere between 20-40 mg in total.
If you plan to use nicotine dihydrogen ditartrate in any sort of preparation, weighing out just the right amount is crucial. Take 10 mg of the salt, and you’re only getting roughly 3.9 mg of actual nicotine. Adjusting formulas without this knowledge risks accidental overdose. Symptoms such as vomiting, headache, or rapid heartbeat shouldn’t ever come as a surprise—you know something has gone very wrong if they do.
Crowdsourcing dosage advice from online forums can backfire. Medical professionals and pharmacists possess both the academic background and hands-on experience to calculate dosages safely and adjust for factors like age, weight, and other health conditions. Anyone trying to use nicotine salts outside of regulated medicine puts themselves at risk—not just from overdose, but from unpredictable reactions, especially when mixing with other compounds.
Lack of transparency about formulation standards and accessible information make missteps more likely. Calling for more public education about nicotine salts and their dosing would prevent confusion and reduce the odds of accidental poisoning. Pharmacies and research institutions already use strict protocols and double checks, often leaning on software and barcoded vials to stop mistakes before they happen. Those steps deserve to become more common, not less.
From personal work in pharmaceutical consulting, I’ve watched how clear, well-written instructions can mean the difference between safety and risk. Transparent dosing guidance, easy-to-follow conversion tables, and reminders about the difference between salt and freebase forms would help not just the doctors and pharmacists, but anyone who comes in contact with these compounds—students, researchers, or tobacco quitters chasing new options.
Nicotine Dihydrogen Ditartrate flies under most people’s radar. Most folks recognize “nicotine” from cigarettes or vapes and forget there are a few other forms out there. Scientists and pharmaceutical companies often use this compound in research or the production of alternative nicotine products. Even though it’s got a fancy name, we’re still talking about nicotine—and that comes packed with its own bag of issues.
People notice a pretty immediate rush after using anything with nicotine, whether it’s tartrate or another salt. That first kick comes from how nicotine hits the brain’s reward system. Suddenly, there’s a rush of dopamine, leading to feelings of alertness or even a slight “high.” But that’s just the start.
Soon, the less pleasant stuff follows: dizziness, headache, nausea, and sweating. The timeline can be quick. Especially for someone not used to nicotine, a single dose can cause an upset stomach, shakiness, or even throw off your heart rhythm. I’ve read more than a few research accounts with participants tapping out of studies early because these side effects grew uncomfortable.
Nicotine puts extra work on the heart and blood vessels. The blood pressure shoots up. The heart thumps faster. There’s a reason doctors ask about smoking before surgery or certain diagnoses—they worry about blood flow and clotting because nicotine restricts blood vessels. At higher doses, like those sometimes used in pharmaceutical trials with dihydrogen ditartrate, some people feel irregular heartbeats—a condition called arrhythmia. That’s a pretty big red flag for anyone with existing heart concerns.
I remember one friend trying to quit smoking with a homemade vape solution, not realizing it included nicotine dihydrogen ditartrate. He landed in the emergency room with his heart racing and a sense of panic. It turned out he’d taken too much, all at once.
It’s no secret: nicotine causes addiction. Structurally, dihydrogen ditartrate behaves like any form of nicotine, pushing people to crave more. That’s where the trap sets in. Over time, constant exposure changes brain chemistry. Memory, attention span, and risk of developing anxiety or depression all take a hit with prolonged use.
And then come the withdrawal symptoms: irritability, trouble sleeping, strong cravings, and feeling mentally sluggish. It’s not easy to get rid of, even for people who want to quit. Studies covering pharmaceutical uses highlight these very points. Not much difference in the struggle whether it’s patch, gum, or pharmaceutical solution.
There’s a lesson here about caution. Anyone handling or experimenting with this compound—scientist, hobbyist, or user—ought to use gloves, goggles, and clear labeling. Accidental skin contact or inhalation can trigger side effects right away. In my own lab work, we stored all nicotine compounds away from high-traffic areas, logged batch numbers, and used neutralizing agents for spills. Mishandling can lead to health emergencies, even at low concentrations.
Policy has its role, too. The FDA and other agencies push for tighter labeling rules. Clear instructions and accessible support make a difference. If someone struggles with quitting or with symptoms, healthcare providers can suggest evidence-based methods: behavioral therapy, approved medications, and social support.
Whether in research or recreational use, respecting nicotine’s risks—especially in concentrated forms—ought to guide every step.
Nicotine dihydrogen ditartrate doesn’t get as much public attention as plain nicotine. Still, anyone who’s worked around it—whether in research or pharmaceuticals—knows a slipup can spell trouble. This salt form lands on the hazardous substance lists for good reason. Both accidental exposure and improper storage raise big safety red flags. Keeping this powder secure and stable isn’t just a best practice; it’s about protecting people, property, and reputations.
I remember the first time I handled nicotine compounds outside of college. The training came packed with warnings: one short cut, one overlooked detail, and you risk everything from chemical burns to toxic inhalation. Many forget that even at small scales, the salt form carries danger like its purer cousin. That’s why simple mistakes, like picking the wrong container or leaving a jar uncapped for “just a minute,” can build up to health scares, environmental headaches, or worse. Overconfidence or sloppy process rarely ends well.
Conditions inside a storeroom make just as much difference as what’s in the bottle. Nicotine dihydrogen ditartrate hates moisture. Humidity in the air will sneak into a poorly sealed jar. Over time, that means degradation, product loss, and unpredictable results in whatever the chemical touches. Nobody wants to repeat tests or recalibrate processes because their chemical stock turned useless. Secure the container tight. Keep it in a cool, dry spot—below 25°C if possible. A climate-controlled cabinet isn’t overkill. It’s good risk management, especially in warmer climates or older buildings where temperature and moisture swing around the clock.
People new to chemical storage sometimes reach for whatever’s handy. But plastic’s a broad field. Some grades turn brittle over time, leach unwanted residues, or simply don’t seal well enough. I’ve found thick, tinted glass jars with Teflon-lined screw caps stand up best over months. Metal can react in rare cases. Thin plastics warp or let air creep in. Invest upfront in the right kind of lab-grade glass, and trouble stays much further away. Label everything—no sharpie on masking tape either. Use chemical-resistant sticks ons or engrave if you’re dealing with larger stocks or shared workspaces.
Regulations differ across countries, but safe storage means preventing unauthorized access, not just spoilage. Lockboxes or restricted-entry cabinets stop curious hands or ill-informed coworkers from getting into something they don’t understand. Training shouldn’t end with your own team. Cleaning crews, security staff, and visiting colleagues should all know they’re looking at more than sugar or salt on the shelf. Sharing incident case studies opens eyes better than any checklist.
No matter how well you store a chemical, nothing lasts forever. Old stock and spills demand just as much respect as fresh powder. Keep a clear protocol for waste collection and handoffs to licensed disposal services. Skip shortcuts like pouring down drains or tossing with regular trash—it’s a recipe for fines or pollution scandals. A safe workplace means full life-cycle thinking from arrival to disposal.
Clear protocols, regular audits, honest training, and solid investment in basic tools all add up to a smaller risk profile. Good storage habits for nicotine dihydrogen ditartrate keep people safe and operations smooth. Letting these basics slide comes with real consequences. Respect the risks, handle with care, and chemical hazards turn from a headache to a manageable part of scientific progress.
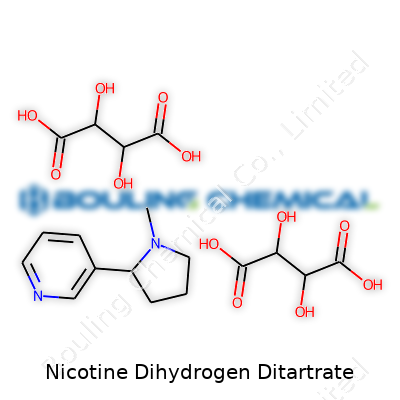
| Names | |
| Preferred IUPAC name | (2S)-1-methylpyrrolidine-2-(pyridin-3-yl)pyrrolidin-1-ium; propanedioic acid |
| Other names |
Nicotine bitartrate Nicotine hydrogen tartrate Nicotinum tartaricum Nicotiana Tabacum tartrate Nicotine ditartrate |
| Pronunciation | /naɪˈkəʊtiːn daɪhaɪˈdrɒdʒən daɪˈtɑːrtreɪt/ |
| Identifiers | |
| CAS Number | [60168-89-8] |
| Beilstein Reference | 3924207 |
| ChEBI | CHEBI:38759 |
| ChEMBL | CHEMBL1201197 |
| ChemSpider | 20301796 |
| DrugBank | DB01322 |
| ECHA InfoCard | ECHA InfoCard: 03dd1a3a-d365-42de-94b7-d57f3c7bc8d5 |
| EC Number | 610-041-6 |
| Gmelin Reference | 38502 |
| KEGG | C05344 |
| MeSH | Ditertrates |
| PubChem CID | 446220 |
| RTECS number | XN8050000 |
| UNII | 7J973YJE8I |
| UN number | UN3316 |
| CompTox Dashboard (EPA) | DTXSID8023025 |
| Properties | |
| Chemical formula | C10H14N2·2C4H6O6 |
| Molar mass | 492.45 g/mol |
| Appearance | White solid |
| Odor | Slightly fishy |
| Density | 1.3 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -1.2 |
| Vapor pressure | 1.04 x 10^-6 mmHg at 25°C |
| Acidity (pKa) | 3.12 |
| Basicity (pKb) | 8.02 |
| Magnetic susceptibility (χ) | -87×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.522 |
| Dipole moment | 2.85 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 354.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -7691 kJ/mol |
| Pharmacology | |
| ATC code | N07BA01 |
| Hazards | |
| Main hazards | Toxic if swallowed, in contact with skin or if inhaled; causes damage to organs; very toxic to aquatic life |
| GHS labelling | GHS02, GHS06 |
| Pictograms | GHS06,GHS07 |
| Signal word | Danger |
| Hazard statements | H301: Toxic if swallowed. H311: Toxic in contact with skin. H331: Toxic if inhaled. H410: Very toxic to aquatic life with long lasting effects. |
| Precautionary statements | P261, P264, P270, P273, P301+P310, P304+P340, P311, P330, P405, P403+P233, P501 |
| NFPA 704 (fire diamond) | 3-2-2-W |
| Flash point | > 145°C |
| Autoignition temperature | 215 °C |
| Lethal dose or concentration | LD₅₀ (oral, rat): 50 mg/kg |
| LD50 (median dose) | 50 mg/kg (rat, oral) |
| NIOSH | WN4725000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Nicotine Dihydrogen Ditartrate: 0.5 mg/m3 (as nicotine) |
| REL (Recommended) | 0.01 mg/m³ |
| IDLH (Immediate danger) | 5 mg/m³ |
| Related compounds | |
| Related compounds |
Nicotine Nicotine Salicylate Nicotine Hydrogen Tartrate Nicotine Polacrilex Nicotine Sulfate |