N-Propylimidazole didn’t show up overnight. Chemists began studying imidazoles almost a hundred years ago, finding them in everything from pharmaceuticals to agricultural fungicides. Some imidazoles changed lives—think about antifungals like clotrimazole. As demand for tuning chemical backbones grew, someone tweaked the imidazole ring by adding a propyl group to the nitrogen, and N-Propylimidazole emerged. I remember a postdoc mentioning how, in the 1980s, specialty chemicals often came out of small team labs, each pushing the limits on what the imidazole family could handle. The drive for new industrial solvents, catalysts, and building blocks powered more targeted synthesis of N-Propylimidazole, especially as researchers looked for more affordable, manageable substitutes compared to more hazardous or volatile analogs.
Today, N-Propylimidazole ends up in research kits, industrial solvents, ionic liquid formation, and specialty synthesis. Ordering a bottle in the lab means looking for purity grades: analytical (over 98%), industrial bulk (around 95%), or custom grades for niche uses. Sometimes, it arrives stabilized to keep air or moisture from messing things up. You’ll spot it in some R&D circles, but the general chemical market rarely stocks this unless you go straight to specialty providers who understand the quirks and the limited shelf life if you don’t store it carefully.
Crystal clear or faintly yellow, N-Propylimidazole pours as a liquid at room temperature—less volatile than its shorter-chained siblings but not as greasy as longer alkyl imidazoles. The odor lands somewhere between sharp rubber and musty solvents; don’t rely on your nose for safety, though. It weighs in at a molecular weight of 124.19 g/mol, has a boiling point around 208°C, and mixes well with water and most organic solvents (except maybe some non-polar ones like hexane). Acidic and basic groups on the ring grab attention from researchers looking for amphoteric molecules—its ability to swap between electron donor and acceptor roles gives it extra flexibility in chemical design.
If you scan a bottle in a chemical storeroom, you will spot a CAS number (14823-98-6) and the formula C6H10N2. Labels usually warn about moderate flammability and the need for storage away from heat and oxidizers. Many labs also flag potential for skin and eye irritation. Material Safety Data Sheets stress chemical gloves and fume hoods, even for small-scale use. Reputable suppliers print purity percentage, impurities (if notable), expiration date, recommended storage temperature, and lot numbers for tracking code compliance.
The classic pathway for synthesizing N-Propylimidazole takes propylamine and glyoxal as raw materials, reacting under mildly acidic or basic settings, followed by cyclization. Some labs tweak the solvents or catalysts, either for better yield or greener chemistry, but most still rely on the original two-step method. One chemist told me they once tried microwave-assisted synthesis to cut reaction times, but product isolation turned messy with too much polymerization side product. After purification, the final product usually rolls out through distillation or liquid-liquid extraction, which also separates residual starting materials and any tars formed during the cyclization.
N-Propylimidazole proves its worth as a modest but flexible nucleophile. You can alkylate, acylate, or sulfonate the ring to create more exotic derivatives. It can grab metal ions, especially in the coordination chemistry space—acting as a ligand for catalytic cycles or metal transport models. In the energetic materials sector, adding nitro or azido groups to the ring helps create highly reactive intermediates. I recall working with a variant where the propyl group let us solubilize a stubborn pharmaceutical salt because the usual methylimidazole just didn’t dissolve it. It reminds us that even minor tweaks to side chains can help tailor properties or unlock new reactivity pathways, especially for chemists looking to sidestep toxic reagents or harsh conditions.
Across catalogs and academic papers, N-Propylimidazole wears more names than you’d think: 1-Propylimidazole, N-Propyl-1H-imidazole, and sometimes just Propylimidazole for short. In patent literature, it sometimes hides behind developer brand codes or obscure trade names, especially in pharma or specialty solvent applications. This creates headaches for anyone cross-checking safety data or sourcing information—double checking CAS numbers keeps things straight. In my own notes, I type “N-Pr-Im” as shorthand, but regulatory filings always want the full formal name.
On the job, working with N-Propylimidazole usually means gloves, goggles, and fume hood running. Even tiny amounts can cause skin redness, itching, or eye discomfort. Spills create slick, smelly puddles—lab floors need to stay dry, and open flames pose burn risks. Standard operating procedures (SOPs) spell out air handling rates and backup spill kits. Chemical incidents with imidazoles in the past two decades made labs build in local exhaust systems, especially near prep benches. Waste gets stored in tightly sealed, labeled drums for pickup, as even trace residues sometimes trip regulatory reporting limits. On a busy shift, simple steps like checking bottle caps or wiping residue from bench tops stopped more close calls than any fancy digital sensors.
N-Propylimidazole doesn’t only belong in academic test tubes. Specialty chemical makers use it as a tailored solvent or as a starting material for ionic liquids prized for their electrochemical stability. Some biotech firms like its structure for enzyme or receptor studies, because the alkyl side chain tweaks cell permeability and protein binding. In polymer science, it acts as a monomer or modifier, producing materials that resist water, offer targeted adhesion, or serve as anti-static coatings. Battery engineers experiment with its derivatives in next-generation electrolytes and corrosion-proofing blends for electronics. My own experience included using it as a phase transfer catalyst for tricky reactions—other amines just didn’t cut it for the substrate at hand.
Interest in N-Propylimidazole ramps up whenever a new field stumbles on an application gap—like ionic liquid chemistry in the late 2000s or in pharmaceutical salt formation two years ago. Research teams dig into ring modifications, tacking on halogens, nitriles, or longer alkyl chains to test for antimicrobial potential or unusual solubility. Recent studies investigated its ability to chelate rare earth metals, aiming for greener extraction methods in electronics recycling. Some biotech startups pursue it as a backbone for enzyme inhibitors or side chain modifications in peptide drugs. You’ll also spot it in conference posters about improving membrane durability or tweaking low-temperature reactivity for next-gen batteries.
Toxicology studies on N-Propylimidazole still trail behind its cousins. Early data points to mild to moderate skin and mucous membrane irritation, with higher doses affecting liver and kidney function in rodents. As labs push for greener credentials, they test these impacts on aquatic life; initial findings show rapid breakdown under sunlight but some bioaccumulation when flushed down municipal waste streams. Regulatory agencies want to know chronic exposure levels, especially as new uses spawn wider industrial handling. The lack of full-scale epidemiological data keeps risk analysts cautious. My personal rule was never to trust a new batch without reviewing safety data anew, as impurities shift based on supplier or manufacturing tweak.
Looking ahead, the future for N-Propylimidazole hinges on tighter green chemistry standards and the search for smarter molecular tools. Battery developers want electrochemically stable, non-volatile solvents, which pushes research into propylimidazoles and their analogs. Biotech startups try to piggyback on the molecule's tunable side chain for enzyme targeting or new biologically active compounds. Researchers also probe new ways to recycle the ring structure, hoping to reduce chemical waste and reclaim spent imidazole stocks from manufacturing. I expect to see more cross-disciplinary partnerships as material science asks for more tailored performance and as the pharmaceutical sector demands safer, more biodegradable scaffolds. Each new discovery seems to rest less on the chemical itself and more on the creative approaches to using its properties—reminding us that even niche molecules can stand out when given the right problem to solve.
N-Propylimidazole sounds like something you’d spot in a textbook, but it pops up in places a lot closer to daily life than many realize. Some of the most unassuming products owe a bit of their effectiveness to this molecule. I’ve run into N-Propylimidazole in the context of manufacturing and lab research, and it always strikes me how subtle but essential its use can be.
In chemical labs, this compound takes on the role of a base or a catalyst, supporting other reactions without taking the spotlight. Paint and coating producers use it to tweak pH, shepherding the mixture towards the right finish and durability. Pharmaceutical researchers like it for whipping up bioactive compounds and specialty drugs. The way it binds with metals makes it handy in analytical labs where impurities can’t hide, helping scientists reveal what else might be lurking in a mixture.
I remember slogging through long synthesis setups for experimental drugs and seeing N-Propylimidazole on the shelf. It’s not a medication by itself, but it helps researchers build complex molecules. It can nudge a reaction forward just enough to cut lab work from three days to one—something anyone looking for new treatments is bound to appreciate. Beyond the bench, it crops up in diagnostic kits, part of the behind-the-scenes mix that helps labs track down disease more accurately.
Anyone who’s ever painted a room knows some brands get it just right. The finish sticks, dries quickly, colors come out strong. Makers sometimes rely on N-Propylimidazole to help bind pigments with the resins, giving that paint the edge in your hallway or classroom. Without this step, final products might peel faster or look washed out. Small details like this steer the way most folks experience paint, even if nobody’s reading the label.
Back in college, I spent time in an environmental chemistry lab, and I got familiar with these kinds of chemical helpers. In testing water samples, certain reactions need a boost to become reliable and precise. Here the N-Propylimidazole acts a bit like a switch, letting scientists spot low concentrations of metals or contaminants. It's no exaggeration to say the trust in your local water often has roots in hidden chemistry like this.
Like most specialty chemicals, there are conversations about safety. Mishandling or using it in excess can introduce hazards, not just for workers, but potentially for ecosystems. Part of the responsibility lies in keeping tight protocols, giving workers the gear and training they deserve, and treading cautiously whenever scaling up new applications. Strong workplace safety culture and scrutiny from regulators help keep this compound on the helpful side of the ledger.
With the world pushing for greener options, the future of compounds like N-Propylimidazole rests on finding new ways to use them with less waste and lower risk. This process can take time, but I’ve seen progress every year, and the science community keeps moving toward safer, smarter chemistry.
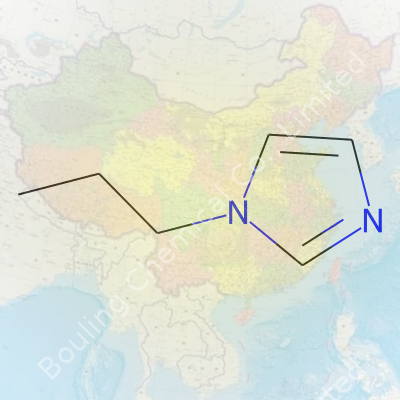
N-Propylimidazole carries the formula C6H10N2. To picture it, start with the imidazole ring, a staple among heterocyclic compounds, then stick a propyl group onto the nitrogen atom. Chemists sketch it out as a five-membered ring with two nitrogen atoms, and the extra propyl chain (–CH2CH2CH3) hanging off the first nitrogen. The shorthand for the structure looks like this:
Imidazole core: C3H4N2 ring — compact, two nitrogens at positions 1 and 3.
Propyl attachment: –CH2CH2CH3 bonded to the N position in the ring.
So, drawing it up, someone’s eye will catch a ring with alternating double and single bonds, two nitrogens spaced out, and then a regular n-propyl chain pointing out from N-1. The result isn’t glamorous but gets the message across.
N-Propylimidazole may sound niche, though people working in a lab will come across it more than they think. I spent time in a university research lab where it played a part in small reactions meant for organic synthesis runs. Imidazole rings show up all over pharmaceutical work, especially when modifying molecules to sharpen how drugs stick to their targets. Adding a propyl group changes how the ring behaves—affecting solubility, how it matches up with metal ions, or slipping it into other molecules for trickier syntheses.
In practice, attaching different side chains like propyl, methyl, or ethyl helps chemists control where and how a certain compound reacts. For example, the propyl group isn’t just decoration: it blocks certain spots from reacting and helps direct reactions in just the way the chemist wants. That small tweak can mean a world of difference, especially in industrial settings or drug design.
Organic chemistry teachers or process chemists often point out that even a small group tacked onto a familiar ring can change a compound’s fate. In N-Propylimidazole, it’s not just a five-ring structure anymore—now the nitrogen’s got a substituent that can make the molecule more greasy (hydrophobic) or sneak into an enzyme pocket in different ways. A compound’s formula and structure shape where it fits into wider chemical families; sometimes, having a linear propyl group instead of something bulkier helps it play a role as a catalyst or intermediate in big production lines.
Changing up the N-substituent also affects how the compound dissolves, how it smells, and sometimes even if it poses risks to health. Some papers highlight how propyl substituents tweak toxicity or absorption rates, which means those small structural details matter beyond the beaker.
Getting your hands on N-Propylimidazole is usually straightforward if you’ve got a connection to chemical suppliers. It pops up as a colorless to pale yellow liquid, with a typical sharp, amine-like odor. It’s not something you splash around casually; it demands safe handling—good gloves, eye protection, and steady airflow. Over the years, I learned to respect even these “simple” chemicals after seeing a friend develop skin trouble from a lapse in laboratory protection. Reading the fine print on the Material Safety Data Sheet before opening a bottle keeps anyone from ugly surprises.
There’s space to improve safety, disposal, and green chemistry around imidazole derivatives. Adding propyl groups could follow greener synthetic pathways—cutting down on hazardous reagents, moving toward more recyclable solvents, and limiting waste. Chemists seeking these alternatives don’t just tick boxes for regulations; they build better lab habits for students and researchers alike. Investing energy here doesn’t sound as glamorous as inventing new drugs, but it keeps everyone safer and lowers the long-term footprint.
So, N-Propylimidazole’s chemical formula (C6H10N2) and its structure offer more than a factoid—they influence how molecules behave, how chemists protect themselves, and which future products hit the market. Details at the atomic level drive real outcomes outside the test tube.
N-Propylimidazole doesn’t show up on most people’s radar unless they work in a lab, a chemical warehouse, or production line. For those who cross its path, it’s not just another name in the SDS sheets—it stands for something that can make a regular workday a whole lot more complicated if it’s ignored. The stuff packs a punch in the organic chemistry world, often showing up in pharmaceuticals, specialty chemicals, and research settings. That reach amps up the importance of handling and storage beyond just ticking boxes on compliance charts.
From my experience, sweating the small stuff when it comes to chemicals like this pays off in safety and sanity. So many incidents trace straight back to skipping basic storage guidelines. People see a clear, colorless liquid and forget: it’s flammable, turning vapor into a hazard zone faster than you realize. Left near an open flame, heating vent, or a careless spark, you set yourself up for trouble. Store it in a spot where temperatures stay cool—preferably under 25 Celsius—out of the direct sun, far from any heat source, and away from oxidizers.
Every lab I’ve ever worked in learns fast: cap your bottles tightly. N-Propylimidazole gives off strong fumes, and it doesn’t take much for vapors to start creeping through loose lids and weak seals. The smell alone tells you something’s up—strong, reminiscent of ammonia, sticking in the air and your memory. Glass containers work well, especially the amber kind that shields the content from light. Metal cans can work, too, but make sure they’re lined with something to keep away corrosion. Forgetting this invites leaks, ruined product, and wasted money.
Forget about grabbing a “handy” container and pouring from the main stock. Unmarked or lazily labeled bottles get people hurt. In shared lab spaces and storerooms, confusion piles up quick when containers drift away from their labels or gather dust without a clear ID. One quick fix? Use bold, chemical-resistant labels and list the full name, hazards, and handle date. Check the shelf life every month; nobody wants a surprise degradation issue or container rupture.
Some folks roll their eyes at PPE until they’re wiping up a splash from their skin or breathing in something nasty. Work with N-Propylimidazole without gloves or eye protection and you risk chemical burns and long, rough days at the clinic. Nitrile gloves, snug-fitting goggles, and flame-resistant lab coats keep accidents from turning ugly. Add a good fume hood into the routine, especially with open transfer or dispensing. Fans and open windows don’t cut it—the vapor can fly right back at you.
Waste streams add extra headaches with this chemical. Open disposal might sound easier, but it’s a recipe for regulatory fines and pollution. I’ve seen barrels fill up with mismanaged leftovers that nobody in the building wants to touch. Pair up with hazardous waste handlers, log every exit and entry, and store used material in compatible, sealed drums while waiting for pickup. Any spill—large or small—requires immediate locking down, absorbent material, and safe ventilation. Watching someone sweep it under the rug (literally) never ends well.
Storing and handling N-Propylimidazole isn’t just following a rulebook—it’s about real people working side by side. The small routines, label checks, and gear choices stack up to protect everyone involved. Slip up, and the costs aren’t just measured in ruined product or missed deadlines—they’re about health and safety, and those bills run high. Everyone’s got a role in keeping it straight, no matter how routine the task seems.
Anyone who spends time in a laboratory runs into names like N-Propylimidazole. On paper, it’s just another compound, but behind the label sits a real question: how safe is it? No one enjoys a hasty reaction from accidental spills, or the anxiety that comes from not knowing the risks. N-Propylimidazole, with its distinct smell and oily feel, isn’t as innocent as it looks on a chemical shelf. Over my years juggling glassware, gloves, and fume hoods, the rule "know your chemicals" keeps showing its value.
Imidazoles in general offer all sorts of uses: pharmaceuticals, corrosion inhibitors, chemical synthesis. The N-Propyl modified version finds its way into reactions because it helps pull off some clever chemical tricks. This convenience comes with a catch. Studies from the National Institutes of Health and the CDC suggest imidazole derivatives often irritate skin and eyes. For N-Propylimidazole, animal research points to moderate toxicity through ingestion and skin contact. Even a brief call to the safety data sheet brings up warning signs: don’t breathe the vapors, watch for splashes, avoid any contact with open skin.
Some people look at hazard statements and think regulators worry too much. Looking back on small lab accidents, you learn the tough way that those warnings come from hard experience. A single spill of an imidazole left rashes on my wrists for days, after gloves tore without me noticing. Colleagues in chemistry circles share stories about blisters and breathing trouble. No one wants to repeat that experience, especially with solvents or reactive compounds like N-Propylimidazole.
The thing with toxicity: it’s not just about “bad or safe.” Dose and contact make the danger. N-Propylimidazole does not top the charts for acute lethality, but chronic exposure isn’t thoroughly studied. Chemical manufacturers mark it as "may cause respiratory irritation" and “harmful in contact with skin.” Unless you have air handling systems and keep up with routine glove changes, frequent exposure builds up risk. No need to wait for a regulatory agency—personal health always matters more than paperwork.
Plenty of labs now lean toward greener chemistry. Reducing use of tricky compounds matters, not only for safety but for keeping waste in check. Imidazole replacements show up more in literature, offering results without the same headaches. I’ve found swapping in less irritating bases works just fine for many reactions. Fume hoods and double gloves turn into everyday habits. Keeping spill kits nearby may feel like over-preparing—until the day someone bumps a flask.
Sitting through safety training as a younger chemist, it sounded like paranoia. Over time, the message stuck: nothing is ever “just another chemical.” N-Propylimidazole illustrates that point. Check the label, wear your gloves, and, if possible, look for less risky alternatives. That extra caution turns nervous caution into careful practice—something you only come to appreciate after spending seasons in the lab. For the next generation, the best lesson comes from seeing others respect both the chemistry and themselves.
N-Propylimidazole often gets overlooked by folks outside the chemical industry. Yet, for researchers and manufacturers, its purity value isn’t just a number – it shapes real results. Standard lab stock usually arrives at purity levels of 97% or higher. Some suppliers go to 98% or even 99%, depending on what the end user wants. From my own time working closely with specialty chemicals, it’s clear that that last percentage point makes a difference, especially if you’re running sensitive syntheses or working with complex drug molecules. Think about a pharmaceutical setting: using raw material at 95% purity versus 99% can change the whole outcome of the process.
Most catalog listings and chemical suppliers label the purity directly: look out for GC, HPLC, or NMR specs on certificates of analysis. Stores and ordering sites offer breakdowns—sometimes you’ll see a surprising range, from technical grade intended for industrial processes, all the way up to high-purity grades reserved for research and pharmaceutical development. Back in graduate school, I learned the hard way what happens if you grab the bulk barrel version (read: lower purity) instead of the clearly labeled “analytical” bottle. Downstream analysis picks up everything you thought you could ignore.
Packaging options for N-Propylimidazole line up with the needs of each industry. The small glass bottle is the classic choice for labs: 25-gram, 100-gram, and 500-gram vials, sealed tightly to keep out moisture and air. Bigger operations often turn to plastic or metal drums, usually 5-kilogram pails or larger. There’s a reason for the variety. Small-scale researchers like to buy only what they need for each run, so smaller bottles make sense. Chemical processors scaling up for production or pilot studies want to avoid restocking headaches, so they go for the drum or container.
Back in a research-grade storage room, there’s plenty of stories about careless boxing and the mess it causes. A leaky cap or a torn seal means hours of cleanup and waste. In regulated industries, tamper-evident seals, moisture barriers, and sturdy caps aren’t just a formality—they actually make life simpler. Bulk drums designed for forklifts or closed transfer systems show up in manufacturing plants more often than you’d think.
Both purity and packaging matter for real safety reasons. While working with N-Propylimidazole, I’ve noticed how easy it is for contamination to creep in if someone overlooks a damaged container or lets an open bottle linger too long. The industry doesn’t rely on strict standards just for show. Tightly controlled seals and pure product help avoid unexpected side reactions, especially in pharmaceuticals, agrochemicals, or electronics.
If you need high-purity stock, always ask for fresh analysis certificates, and push suppliers on expiration dates. For packaging, it pays off to match order size with actual usage. I’ve seen too many labs buy a bulk drum and only use a fraction before it spoils. Splitting bulk product into smaller, clearly labeled containers right away helps, and cuts down on safety risks too.
Manufacturers could offer clearer purity benchmarking and inventory tracking. Some suppliers now print QR codes linked to real-time documentation—easy to check on the fly. Smaller packaging formats would help for one-off projects, while larger drums stay useful for those doing heavy production. These shifts don’t just help specialists or big labs—they save money, reduce chemical waste, and keep workspaces safer for everyone involved.
| Names | |
| Preferred IUPAC name | 1-propyl-1H-imidazole |
| Other names |
1H-Imidazole, 1-propyl- 1-Propylimidazole N-Propylimidazol 1H-Imidazole, N-propyl- |
| Pronunciation | /ɛn-ˌproʊpɪl-ɪˈmɪdəˌzoʊl/ |
| Identifiers | |
| CAS Number | 16729-14-7 |
| Beilstein Reference | 1858736 |
| ChEBI | CHEBI:51152 |
| ChEMBL | CHEMBL13840 |
| ChemSpider | 16686 |
| DrugBank | DB08708 |
| ECHA InfoCard | 03fbd4db-20f8-4e37-8c7c-425c8f64e8f7 |
| EC Number | 211-219-5 |
| Gmelin Reference | 72943 |
| KEGG | C18673 |
| MeSH | D019273 |
| PubChem CID | 69720 |
| RTECS number | UJ5950000 |
| UNII | D2CIPE8Q0U |
| UN number | UN2810 |
| CompTox Dashboard (EPA) | DTXSID70820950 |
| Properties | |
| Chemical formula | C6H10N2 |
| Molar mass | 110.16 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | amine-like |
| Density | 0.992 g/mL |
| Solubility in water | soluble |
| log P | 0.38 |
| Vapor pressure | 0.37 mmHg (25°C) |
| Acidity (pKa) | 14.2 |
| Basicity (pKb) | 5.69 |
| Magnetic susceptibility (χ) | -59.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.484 |
| Viscosity | 3.25 mPa·s (25°C) |
| Dipole moment | 4.48 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 238.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | –75.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3880 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | Harmful if swallowed. Causes severe skin burns and eye damage. Harmful if inhaled. |
| Precautionary statements | P280, P261, P305+P351+P338, P337+P313, P304+P340, P312 |
| Flash point | 44 °C (111 °F; 317 K) |
| Autoignition temperature | 415 °C |
| Explosive limits | Explosive limits: 2–12% |
| Lethal dose or concentration | LD50 oral rat 780 mg/kg |
| LD50 (median dose) | LD50 (median dose): 1240 mg/kg (rat, oral) |
| NIOSH | NIOSH: RX2625000 |
| REL (Recommended) | 0.05 ppm |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Imidazole 1-Methylimidazole 2-Methylimidazole 1-Ethylimidazole 1-Propylimidazole N-Butylimidazole |