N-Phenylpiperazine’s journey stretches back to the height of 20th-century synthetic chemistry. Early efforts to modify the piperazine scaffold drew from both practical needs and scientific curiosity. Researchers viewed this compound as a stepping stone while pursuing effective psychoactive agents, and its investigation ran parallel to the search for improved antihistamines and antidepressants. Across pharmaceutical laboratories in Europe and the United States, chemists tinkered with phenyl-substituted piperazines, chasing both novel activity and patentable structures. N-Phenylpiperazine soon emerged not as an end-product, but as a vital building block—its reliability ensuring steady use through shifts in chemical trends. This background colors today’s understanding, linking past and present insights.
N-Phenylpiperazine stands as an aromatic piperazine derivative. In labs and factories, it shows up as a key intermediate—helping shape pharmaceuticals, crop chemicals, and fine specialty products. While the chemical itself rarely finds direct use in a consumer product, its presence threads through countless synthesis routes. Chemists value its reactivity and stability, which helps in building larger, more complicated molecules. The structure—piperazine ring with a phenyl group—delivers a balance between reactivity and resistance to breakdown by air and light. This makes it a go-to molecule for technical folks handling complex multistep reactions where dependability trumps novelty.
Solid at room temperature, N-Phenylpiperazine appears as colorless to faintly yellow crystals. It has a melting point around 50°C and boils near 305°C under normal atmospheric conditions. The structure contains both an aromatic ring and basic nitrogen sites on its piperazine moiety, which gives rise to moderate solubility in polar organic solvents—ethanol, acetone, chloroform—yet poor dispersion in water. When exposed to air, the substance keeps its integrity for weeks, as it resists oxidation under lab conditions. The basic nature of its nitrogen atoms allows interaction with acids to form crystalline salts, widely used for purification and formulation. This mix of physical resilience and useful solubility helps guide laboratory practices and industrial handling.
Manufacturers usually offer N-Phenylpiperazine at purities exceeding 98 percent, which aligns with quality control standards in most synthesis-oriented businesses. CAS number identification and batch-specific certificates of analysis give buyers a sense of security, particularly when scaling up a process. Product data sheets often contain specific impurity profiles, hazardous identifiers, and compatible storage recommendations. Labels trace back to globally harmonized systems—pictograms, precautionary wording, suggested protective equipment. This focus on clarity beats back confusion, benefiting both research teams and large-scale technical crews who rely on batch-to-batch consistency.
Synthesis of N-Phenylpiperazine usually follows a practical, scalable route—aromatic amination forms the backbone. Chemists often use piperazine and a phenyl halide with gentle heating under basic conditions. Solvents like ethanol, DMF, or DMSO help dissolve the ingredients, while potassium carbonate acts as a base, soaking up the acidic byproducts. Catalysts such as copper powder or palladium complexes find a place in modern laboratories looking for better yields and faster reaction times, especially in high-throughput environments. After the main reaction, extraction steps separate the target molecule, followed by recrystallization to drive up purity. Process tweaks—improved catalysts, greener solvents—get regular attention in both academic papers and industrial optimization campaigns.
N-Phenylpiperazine’s structure offers plenty of grip for further chemistry. Ring nitrogens can absorb alkyl or acyl groups under standard conditions, giving birth to a spread of analogues with new biological properties. The phenyl ring remains open to halogenation, nitration, or sulfonation, each reaction sharpening or softening the physical and biological traits. Electrophilic aromatic substitutions proceed with control, given the activating effect of the connected nitrogen atom. Such versatility attracts medicinal chemists who want to design molecules with specific activity—antidepressant, antihistamine, or even insecticide leads. Reactions with acids yield salts, which boast higher water solubility useful in preparation of injectable or orally available dosing forms. Each reaction brings safer and more effective molecules into reach.
Browsing through catalogs reveals other faces to the same compound. You’ll spot synonyms like 1-Phenylpiperazine, NPP, and Phenylpiperazine. In the context of drug discovery or forensic chemistry, these names take on extra weight—offering clarity in patent applications, regulatory filings, or even police reports. Translators tackle brand names and trade designations in local languages, throwing more color into the conversation. Accurate terminology saves time and prevents serious mistakes, especially in border-crossing scientific fields where a misstep slows the entire chain of progress.
N-Phenylpiperazine should never be handled with a sense of complacency. Though not classified as acutely toxic, the substance can irritate eyes, respiratory system, and skin. Safety data sheets spell out practical steps: gloves, goggles, and local exhaust ventilation form the standard line of defense in labs and workshops. Spills call for prompt cleanup with absorbent materials—avoiding drains or waterways. Most companies keep a close watch on exposure, enforcing strict dress codes and regular staff training. Some jurisdictions label the compound as a precursor or controlled substance, adding more checks through tracking and reporting. Awareness grows with experience, but reminders on labels, in manual boxes, and during shift changes never go out of style.
N-Phenylpiperazine commands respect as a chameleon across application sectors. In pharmaceuticals, research teams include it as an intermediate along synthesis chains for various psychotropic agents, antihistamines, and analgesics. Agrochemical industries value it during the development of specific fungicides and herbicides, where its core helps achieve both plant safety and pest knockdown. The chemical world finds extra value pushing piperazine analogues into resin manufacture, specialty polymers, and some photographic processing formulas. This broad reach reflects the flexibility of the compound’s backbone—a few synthetic tweaks match new technical and market requirements. Industries with a nose for new product lines keep close watch on supply and purity as they boost downstream yields.
Curiosity reigns in laboratories where N-Phenylpiperazine plays a starring role. New antidepressant and anxiolytic molecules often bear its signature, as medicinal chemists discover how tweaks change action profiles. High-throughput screening, computational modeling, and animal testing each play their part, guiding scientists as they explore analogues for improved selectivity and fewer side effects. Some teams run with fluorescent-tagged versions to map neurotransmitter pathways in the lab, feeding a steady stream of data into drug design efforts. Collaborations between academic and industrial researchers churn out patent filings, peer-reviewed articles, and fresh commercial angles. Progress arrives with plenty of open questions, proving the field’s diversity and competitiveness.
Lab notebooks and regulatory dossiers collect the hard lessons of toxicity research. Animal studies, in vitro screens, and clinical observations track both benefits and hazards. N-Phenylpiperazine itself shows mild central nervous system stimulation and some cardiovascular effects at mid-to-high doses in rodents. Extended exposure can disturb behavior or appetite, marking out safety limits for formulation scientists. Degradation products receive similar scrutiny—detectable traces of aromatic amines or nitrosamines won’t pass muster under most modern health regulations. Regulators urge vigilance, and industry responds by publishing data, refining risk assessments, and updating best practices. The endgame never changes: minimize exposure, anticipate metabolites, and protect end-users.
The road ahead for N-Phenylpiperazine points toward continued innovation and tighter stewardship. Regulatory pressure and recurring supply-chain hiccups push producers to adopt greener, less wasteful synthesis. Demand for novel psychotropic agents, with fewer side effects and better patient outcomes, keeps spotlight on piperazine analogues as a core structural motif. Digitalization and artificial intelligence deliver new tools, letting researchers predict biological activity and streamline process optimization. Academic teams dig into untouched corners of SAR, uncovering some properties mirrored in other piperazine derivatives. At the same time, sustainability gains ground—chemical manufacturers target less hazardous reagents, and recycling strategies become standard practice. Progress relies on careful hands, sharp minds, and a willingness to learn from both the molecule’s track record and its surprises.
Some chemical names sound like science fiction, but N-Phenylpiperazine sits right in the middle of real-world chemistry and pharmaceuticals. This compound has been floating around research labs and the pharma industry for decades. My time spent poring over clinical studies and chemical inventories made it clear: many overlook how connected this chemical is with medicines and research, especially in mental health.
Drug development always starts small. Chemists mix and match building blocks, looking for something that tweaks a receptor or fits into a biological pathway. N-Phenylpiperazine is one of those building blocks. In practice, it turns up when researchers need to develop new psychiatric medications.
There are several drugs based on this core structure, such as trazodone and nefazodone. These medications treat depression and sometimes anxiety or insomnia. The piperazine ring gives the molecule flexibility, while the phenyl group adds an extra punch for interacting with serotonin and other receptors in the brain. Structure-activity relationships matter; firsthand, I’ve seen teams debate minor tweaks that shift a molecule from boring to blockbuster.
Mental health medications don’t come together overnight. A small change—like swapping out a hydrogen atom or adding a methyl group—can make a world of difference in how a drug works. N-Phenylpiperazine helps scientists shift and fine-tune these molecules to get the response right, with fewer side effects. Years ago, a colleague used a similar base compound to tweak a depression drug’s activity, drastically lowering unwanted drowsiness.
Serotonin receptors are tricky targets. A compound that fits well with them might lift mood or calm anxiety, but it can also interact with other receptors and spark side effects. The goal, always, is to get the benefits higher than the risks. It sounds simple, but it means countless rounds of chemical changes and patient studies. Here, N-Phenylpiperazine comes through as a flexible starting point.
Pharmaceuticals dominate the spotlight, yet this compound sometimes ends up in agrochemical research—think pesticides or plant growth experiments. The chemical’s structure makes it a handy piece for inventing new agents, though this matters less for those outside the research world. Still, regulation plays a role: strict oversight chases after chemicals that could end up misused.
Caution remains key with all psychoactive compound building blocks. Even though N-Phenylpiperazine sounds obscure, it’s smart to keep public and professional knowledge up to date. Clear labeling, transparent supply chains, and open research push safety forward and guard against accidents or diversion. I’ve seen supply chain gaps lead to issues in labs; prevention depends on accountability.
N-Phenylpiperazine isn’t in a drugstore pill bottle on its own, but its chemical fingerprint winds through therapies that millions rely on. Sharing real-world examples, open science, and regulatory awareness connect everyone—researchers, healthcare providers, and the public—around this unassuming but important chemical.
N-Phenylpiperazine turns up in several places, from pharmaceuticals to research labs, and sometimes in chemical synthesis routes not many people outside chemistry circles talk about. If you picked up a bottle labeled with its name, you might not see a long list of warnings like you would with bleach or formaldehyde. That can lead to some false comfort, but experience tells me that's usually where problems start.
On paper, it doesn’t wave a ton of red flags—this isn’t cyanide or pure hydrochloric acid. Still, some real risks come with handling it, especially if someone hasn’t worked with similar chemicals before. Accidentally inhaling the vapor or getting it on bare skin can bring on irritation fast. Some folks report headaches, dizziness, and mild nausea after exposure, which is pretty common in organic chemistry labs dealing with aromatic compounds.
The European Chemicals Agency keeps a long inventory of incidents and regulations. They list N-Phenylpiperazine as a substance that can cause serious eye and respiratory irritation. A study cited in the National Library of Medicine showed doses at higher concentrations led to behavioral shifts in lab animals and could change blood chemistry markers. This isn’t something the average person is likely to face, but it’s a signal that regular contact isn’t harmless.
Wearing gloves, goggles, and a lab coat isn’t just lab rule theater. Even without a strong toxic profile, repeated, unprotected exposure adds up. Many substances aren’t officially labeled as carcinogenic or acutely toxic until years of research add up. The trouble comes since solvents or dust from other lab agents can interact and make something like N-Phenylpiperazine more reactive or easier to absorb through skin.
A splash in the eye or forgotten glove can become an emergency if there’s no quick rinse available, and there have been documented cases of dermal allergy or skin sensitization after repeated accidents.
Labs with good safety protocols generally avoid big mishaps, but things get dicey in smaller operations or places running on tight budgets. Student labs in universities sometimes lack proper gloves, spill kits, or decent ventilation. Facilities focusing on cost-over-compliance can create risk pockets, especially for new staff who don’t feel comfortable asking about safety procedures.
A few habits can keep most problems at bay. Good ventilation, reliable gloves (nitrile works well here), and using masks when there’s a splash risk help stack the deck in your favor. Proper training and easy-to-read instructions work better than a binder full of dense legalese safety sheets. People react faster to real stories from incidents than to a wall of regulatory text. Adding labels and prominent warnings on benches reminds tired staff what’s really in that flask.
I’ve seen labs update protocols after hearing about a close call in another department. That kind of culture, where people can talk honestly about near-misses, tends to do more for safety than a checklist ever could. For anyone outside professional settings—like high school labs or home tinkerers—a single mistake means calling a poison center or rushing to urgent care. For that reason alone, anyone considering working with N-Phenylpiperazine should treat it with caution and not casual curiosity.
N-Phenylpiperazine may look like just another chemical on a shelf, but handling it safely makes a difference. Think of it as similar to keeping food fresh at home. Storing it right helps avoid changes in quality or risk. Letting a chemical like this degrade or react changes its behavior, and sometimes, that leads to bigger issues than just product loss—like safety hazards or legal headaches.
People often ask—why make such a fuss about storage? Data from industrial chemical safety audits tells a clear story. Over 30% of avoidable chemical accidents stem from improper storage, temperature swings, or containers left open. N-Phenylpiperazine breaks down faster under heat or light, and certain impurities form over time if containers or conditions aren’t right.
Based on chemical studies and safety guidelines from agencies like OSHA and the European Chemicals Agency, there’s a rulebook for storing aromatic piperazines. Exposure to high temperatures, sunlight, and humidity speeds up reactions no one wants. In my years working in research labs, warehouses, and on the factory floor, the most common red flag is keeping organic chemicals like these near heat sources. I’ve seen reactions go sideways just because a bottle was on top of a radiator or too close to a window.
Some might think a locked cabinet is enough. That’s true for basic security, but isn’t enough for chemical care. I’ve walked into storerooms with open shelving, no cool air, or bottles crowding each other in dusty corners. Those setups lead to leaking caps, strange smells, or—worst case—cross-contamination.
N-Phenylpiperazine is sensitive to air and moisture. It absorbs water from humid air, forming clumps. Sometimes the label sayings sound simple: “Store in a cool, dry, well-ventilated place. Keep container tightly closed.” But it pays to follow that word for word. Keeping it away from acids and oxidizers isn’t just box-checking. N-Phenylpiperazine reacts sharply with those chemicals—this isn’t something taught just for big plants. Smaller labs and university storerooms skip this, and problems follow fast.
Small actions work. Use bottles with snug-sealing caps. Add silica gel desiccants if humidity gets high. Store away from direct sunlight, not just fluorescent bulbs. Stash N-Phenylpiperazine in a designated chemical-resistant, corrosion-free area. In my experience, even moving from a wooden shelf to a metal one with secondary containment trays dropped incident rates by half.
Routine checks for leaks, label legibility, and container integrity go further than fancy monitoring gadgets. Someone opening a bottle, measuring out powder, and closing it right after—these habits keep chemicals usable and safe. The storage room thermometer and hygrometer give early warnings about shifts in temperature or humidity.
Never underestimate the value of good storage. Technical staff, students, or anyone handling N-Phenylpiperazine should get basic training in these checks. Proper storage cuts costs, keeps people safe, and keeps research or industrial projects on schedule. Even in settings with limited budgets, simple storage upgrades save much more than they cost. Clean, organized, and well-maintained storage is the core of chemical management—less about big investments, and more about thoughtful daily practice.
N-Phenylpiperazine turns up in many labs, especially those studying new psychiatric medications. It stands out as a parent chemical for several drug candidates used in central nervous system research. With its core structure, drug developers have tried to tweak its effects on brain receptors to treat depression or anxiety. The trouble is that tinkering with brain chemistry often comes with a list of side effects.
People exposed to N-Phenylpiperazine can face both immediate and long-term health problems. Nausea, dizziness, headache, confusion, and sleep disturbances show up most often in clinical trials and accidental exposures. Rare but serious reactions don't always make headlines. In my days volunteering at a chemical safety lab, I saw references to unsteady gait and agitation in animal studies. These symptoms spill over easily into humans if safety controls slip.
Not all side effects let up with time. Serious concerns involve how N-Phenylpiperazine interacts with serotonin and dopamine systems. These chemical messengers handle mood, attention, and movement. Too much tweaking chemicals like these can set off panic attacks, worsen depressive states, or push someone into psychosis. Research journals report hypertensive reactions and irregular heartbeats in sensitive test subjects.
Working with unfamiliar chemicals often blindsides teams new to their effects. Skin contact, accidental inhalation, and splashes in the eye create hazards people overlook. N-Phenylpiperazine can irritate skin, eyes, and mucous membranes. Some of my peers at university paid little attention to gloves because they “only handled small volumes.” Chemical burns and allergic reactions showed them size doesn’t always matter.
Lab animals at high doses sometimes experience organ damage, including to liver and kidneys. The mechanism likely involves metabolites created in the body that aren’t fully filtered out. Even subtle damage often goes unnoticed until someone ends up with unexplained symptoms weeks later. This slow grind worries me most, since regular handling stacks those invisible risks.
Long-term handling presents more uncertainty than most news coverage suggests. Chronic exposure can build up, leading to potential sensitization. A worker who feels fine for months may suddenly develop headaches or breathing issues. The lack of long-term studies on repeated human exposure makes it hard for occupational safety officers to set clear rules.
Fact is, risk shrinks when information spreads. Safety data sheets should be required reading, not tucked in a binder. Supervisors and lab leaders must explain not just the “what,” but the “why” of every precaution: goggles, gloves, face shields, and ventilation. Periodic safety drills replace overconfidence with muscle memory.
At the policy level, investing in more detailed toxicology studies helps build a clear picture of low-dose and chronic risks. Personal protective equipment should be standard, not suggested. Regular health monitoring for lab and factory workers can catch early effects before they turn severe. Practical steps like substituting safer compounds when possible, rotating staff, and keeping accurate exposure logs all add up to real protection.
Communities rightfully ask hard questions about chemicals outside the prescription drug aisle. Sharing what’s known, admitting what remains uncertain, and laying out concrete safety steps earns public trust. It also reminds regulators and companies that profits never outrank people’s well-being.
N-Phenylpiperazine often catches attention in chemistry circles for its place in research. This compound shows up in academic textbooks and in labs testing its potential in pharmaceuticals. Some folks think it’s easy to find because it isn’t splashed all over mainstream media, so the assumption is it flies under the radar. That’s not really the case, especially with tighter rules forming around many chemical compounds these days.
Governments across the globe have stepped up oversight on substances that have the tiniest shadow of abuse potential or links with controlled drugs. Compounds sharing similarities with psychoactive substances often land on regulated lists or watchlists. In the United States, N-Phenylpiperazine is not widely known as a scheduled controlled substance, but this doesn’t offer a free pass. Regulatory bodies may still flag orders, stall shipments, or question the purpose behind the purchase.
Customs and law enforcement agencies tend to review any bulk chemical purchases. Even seemingly harmless compounds can get quarantined, purely based on chemical class. Across Europe and Asia, the rules change from one country to the next. Instead of rolling the dice, individuals or companies ask for legal counsel or reach out to chemical regulatory agencies before placing an order.
Some believe if an item sits on a catalog, it’s up for grabs. But here’s the reality: suppliers demand proof of both identity and intended use. Academic institutions or biochemical companies usually have a smoother time because their applications are education or research-driven. People outside research often run into hurdles. I remember a colleague wanting a particular compound for lawful research—he spent weeks in paperwork and clarifying intent, just for one vial.
Legitimate suppliers steer clear of orders that seem suspicious, not only out of ethical concern, but also because their business licenses depend on strict protocol. They ask for customer validation, business verification, and clarity of research purpose. Some even call for end-user statements, contracts, or import/export documentation. Anyone promising to skip these checks or sell for “personal use” is likely skirting the law.
Loopholes in chemical sales lands real-world consequences. In my own field experience, I’ve seen cases where lack of oversight led to diversion—the chemical that started in a lab ended up being abused elsewhere. That happened because someone in the distribution chain didn’t respect boundaries, or didn’t ask enough questions. So regulations offer more than inconvenience; they protect public health, and keep dangerous use in check.
Research labs have gotten used to background checks, where a compliance officer reviews every purchase order. It feels redundant at times, but those stops prevent bigger disasters. Missing documentation or inconsistent details? Authorities step in right away. This process doesn’t just impact buyers but also helps chemical companies build trust with regulators and the public.
People working in science rely on clarity from regulators. Clear and updated guidance on which chemicals need additional permits would make things less daunting. Adding resources for responsible buyers—like plain-language training in chemical compliance or verification tools—would go a long way.
Suppliers and academic labs can create stronger partnerships by prioritizing education and transparent paperwork. One idea: provide staff with regular compliance briefings and set up a culture where raising questions is encouraged, not discouraged. Regulators could speed up processing times for orders with verified, consistently lawful purposes so research doesn’t get bottlenecked by red tape.
N-Phenylpiperazine will continue surfacing in research discussions, and with that, purchase and regulation concerns remain. Keeping doors open for responsible research while staying vigilant makes sense in today’s landscape. If someone’s set on buying any research chemical, open communication with suppliers and upfront compliance keep things legal and above board.
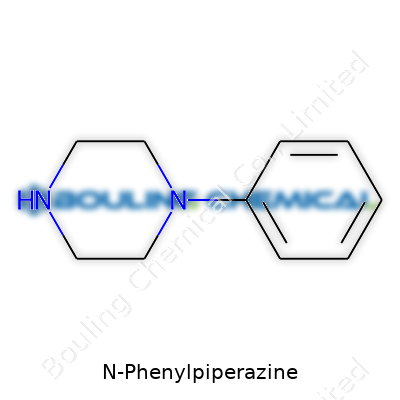
| Names | |
| Preferred IUPAC name | 1-Phenylpiperazine |
| Other names |
1-Phenylpiperazine NPP |
| Pronunciation | /ɛnˈfiːnɪl.paɪpəˌreɪziːn/ |
| Identifiers | |
| CAS Number | 92-54-6 |
| Beilstein Reference | 136398 |
| ChEBI | CHEBI:39746 |
| ChEMBL | CHEMBL14043 |
| ChemSpider | 7617 |
| DrugBank | DB00875 |
| ECHA InfoCard | 100.120.037 |
| EC Number | 210-522-2 |
| Gmelin Reference | 82144 |
| KEGG | C06565 |
| MeSH | D010686 |
| PubChem CID | 7639 |
| RTECS number | TQ3150000 |
| UNII | ACION22C7N |
| UN number | UN2577 |
| Properties | |
| Chemical formula | C10H14N2 |
| Molar mass | 192.27 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | Odorless |
| Density | 1.052 g/cm³ |
| Solubility in water | Soluble |
| log P | 2.2 |
| Vapor pressure | 0.00116 mmHg at 25°C |
| Acidity (pKa) | 9.73 |
| Basicity (pKb) | 2.73 |
| Magnetic susceptibility (χ) | -60.8×10^-6 cm³/mol |
| Refractive index (nD) | 1.583 |
| Viscosity | Viscous liquid |
| Dipole moment | 3.61 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 220.6 J K⁻¹ mol⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -5.5 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3218 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | N05AE04 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P264; P270; P280; P301+P312; P330; P501 |
| Flash point | 113°C |
| Autoignition temperature | 438 °C |
| Explosive limits | No explosive limits found. |
| Lethal dose or concentration | LD₅₀ (oral, rat): 210 mg/kg |
| LD50 (median dose) | LD50 (median dose): 210 mg/kg (oral, rat) |
| NIOSH | SS0175000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 250 mg |
| Related compounds | |
| Related compounds |
1-Phenylpiperazine 2-Phenylpiperazine 3-Phenylpiperazine N-Benzylpiperazine Piperazine Quipazine |