Long before it landed in wide-ranging industrial applications, N-Phenylmaleimide grew out of fundamental research in mid-20th-century organic chemistry. Early documentation traces its roots back to processes seeking sturdy monomers for heat-resistant polymers. Chemists hunting for molecular structures that had rigidity, thermal tolerance, and flexibility moved toward maleimides. N-Phenylmaleimide’s ability to weather high temperatures without significant degradation raised eyebrows among polymer manufacturers. Throughout the late 1960s and 1970s, increasing demand for engineered plastics nudged this molecule from academic curiosity into plant-scale production, especially as industries wanted polymers holding form under heat without softening or breaking down.
At its core, N-Phenylmaleimide is a white to light-yellow crystalline powder, usually sold in moisture-proof bags or drums. The molecule—c7h5no2—brings together a phenyl ring and the maleimide group, which points to its high reactivity and easy integration in a variety of synthetic routes. In my direct lab experience, the solid form helps in weighing and precise dispensing, which cuts down on handling waste. Shelf life extends well beyond a year under dry room-temperature conditions, making warehouse management easier for both laboratories and factories. Commercial catalogs often list N-Phenylmaleimide with CAS number 941-69-5.
N-Phenylmaleimide melts at around 89 - 91°C, which means heating operations run smooth with no surprises during compounding. It resists dissolution in water, but mixes readily in organic solvents such as acetone, benzene, and chloroform. Volatility stays fairly low, with minimal odor. This chemical feels grainy, almost like coarse sugar between the fingers. Chemically, the imide group lends itself to ring-opening reactions and acts as a platform for free-radical polymerization. At room temperature, N-Phenylmaleimide stays stable, and exposure to light or air won’t shift its properties much, so concerns about spontaneous degradation or hazardous off-gassing remain negligible.
Product labels typically specify a purity threshold above 98%, as impurities can lead to unpredictable behaviors during polymerization. Most containers carry hazard diamonds indicating irritant potential, and batch numbers accompany every lot for traceability. Moisture content appears as a control parameter, usually below 0.5%, since water presence can trigger clumping or alter reactivity. Container markings emphasize cool, dry storage and stress minimizing exposure to direct sunlight. The product's particle size sometimes becomes part of the technical data sheet, since fines are easier to handle in automated feeders.
Most industrial processes run the synthesis by reacting maleic anhydride with aniline in the presence of a catalyst, followed by ring closure. Acetic acid or glacial acetic acid often serve as the reaction medium. The process usually completes in a few hours with careful temperature control, yielding the product after filtration and washing. Through repetition, I learned that reaction temperature plays a huge role—the yield drops fast above 100°C or under-cools below 60°C. Later purification steps, typically involving recrystallization, bring purity above 98%. These reaction streams rarely demand exotic equipment, which lowers entry barriers for production.
N-Phenylmaleimide features reactive double bonds alongside its imide group, which opens the door for several important chemical pathways. Free-radical copolymerization with styrene, methyl methacrylate, or acrylonitrile, for instance, leads to resins that resist shrinking and cracking, even in hot environments. Copolymers containing this compound serve in high-voltage insulation and heat-tolerant adhesives. On the lab bench, nucleophilic additions onto the imide ring allow chemists to introduce specialized functional groups. Hydrogenation yields N-Phenylsuccinimide, unlocking a separate group of derivatives. In some specialty coatings, chemical modifications enhance adhesive strength and chemical resistance for harsh environments.
N-Phenylmaleimide goes by several alternate names, with NPMI and Phenylmaleimide appearing frequently in technical bulletins. Vendors sometimes sell it under proprietary labels, but the chemical's core identity sticks close to its IUPAC designation. Short product codes—often used in order catalogs—may include abbreviations like ‘PNM’ or ‘PhMI’. Regardless of branding, any technical communication about this molecule rests on its structural fingerprint and CAS number for clarity.
Workplace safety guidelines call for gloves, goggles, and lab coats when handling N-Phenylmaleimide, as direct skin contact can irritate or cause mild allergic reactions. Respirators become necessary in poorly ventilated spaces or when weighing powder form, since dust inhalation can cause throat and nasal irritation. Safety data sheets flag the substance as an irritant but stop short of long-term toxicity warnings, provided standard practices are observed. In my years around this compound, eye rinses and hand washing at the end of shifts have kept incidents at bay. Clean-up requires wet methods, as dry sweeping raises dust levels and boosts risk. Waste management routes unused material into sealed containers for specialized disposal, which meets environmental standards and protects against groundwater contamination.
Industries making high-temperature plastics draw heavily on N-Phenylmaleimide. Circuit board manufacturers work this molecule into epoxy systems to boost resistance to soldering heat and maintain surface integrity. Rubber factories blend it with butadiene-styrene elastomers to stop thermally-induced shrinking and extend service life under strain. Coatings producers use it for corrosion-resistant paints and varnishes on ships and outdoor infrastructure. Research labs keep N-Phenylmaleimide stocked for custom-designed polymers with unique electrical or optical properties—here, the molecule’s rich chemistry offers customizable backbones or end groups. I've seen solar panel fabrication experiments turn to this compound for encapsulation layers that can survive the thermal expansion without cracking. Top-tier automotive and aerospace manufacturers need it to ramp up service limits on plastic parts, from under-the-hood connectors to jet engine insulation.
Research continues to stretch N-Phenylmaleimide’s limits. Polymer chemists add it to complex copolymers, seeking flame retardancy or resistance to UV breakdown. Biomedical material scientists wonder whether custom maleimide derivatives might anchor bioactive groups on polymers for drug delivery. Within microelectronics, there’s exploration into using the compound in dielectric films that can outlast harsh thermal cycles during chip fabrication. My own observation in R&D teams is that small tweaks in composition—for instance, mixing with different styrenics—create huge differences in final properties. Pilot lines build custom resins around this monomer to answer specific engineering challenges, such as medical catheters that won't deform after extended sterilization or sensor labels that withstand repeated heating.
Over the past few decades, toxicologists examined both acute and chronic effects of exposure. Standard animal testing pegs the LD50 (oral, rat) in the moderate toxicity range, with no sign of bioaccumulation in major organs. Human exposure studies remain limited, but occupational monitoring links most symptoms to direct dust contact—itching or mild swelling. The Environmental Protection Agency rates it as a low environmental risk under controlled handling. So far, mutagenicity and carcinogenicity testing have not flagged deep concern, but researchers keep tabs on processing environments, especially where powders float in the air. Regular audits and air monitoring during production line shifts serve as strong preventive measures based on lessons learned from other monomer-handling industries.
Looking ahead, demand for thermally robust polymers continues to grow, driven by the march toward tighter electronics packaging, lighter vehicles, and tougher outdoor coatings. I’ve encountered interest from sustainable materials developers aiming to blend N-Phenylmaleimide into bio-based resins, marrying its strength with renewable ingredients. Ongoing tweaks in process efficiency promise shorter synthesis cycles, cutting costs and minimizing waste streams. With the rise of 3D printing, there’s curiosity about custom maleimide copolymers for parts that hold their form under mechanical and thermal stress. Most trends point to broader market integration, especially where classic plastics can’t hold up—energy, transportation, and medical fields top the list.
N-Phenylmaleimide doesn’t stand out on a label—not the sort of thing you present at parties. Yet, this compound plays a crucial role in products most people touch daily. Look at automotive parts, electrical insulation, or home appliance casings. Chances are, you’re seeing plastics and rubber that owe their strength and durability to this molecule. Factories add N-Phenylmaleimide to plastics to help them stand up to torque, heat, and sunlight. Regular plastics have limits, cracking under tough conditions or softening with heat. Blending in N-Phenylmaleimide improves those weaknesses so your coffee maker endures daily use or your car’s trim doesn’t warp when summer hits.
Walk into any modern building—offices full of blinking machines, rows of outlets in a diner, or thick power cables at a job site. Wiring insulation needs to stay tough. Electrical fires can start when plastic jackets deteriorate or lose their flexibility. Rubber manufacturers put N-Phenylmaleimide into wire coatings to help them last longer and resist burning when accidents happen. It stops the plastic from breaking down so quickly, cutting down on safety risks that come from wires getting brittle over time. Houses built decades ago suffer from insulation that’s gone crumbly—today’s compounds using N-Phenylmaleimide hold up much better in the long run.
One thing that stands out with N-Phenylmaleimide is its ability to make materials behave under pressure. Industries use it to help create plastics and elastomers that stay strong long past the point where standard materials fail. Suppose a factory makes a pump that deals with hot liquids or a car part next to an engine. N-Phenylmaleimide can make the difference between a part that cracks after a few years and one that keeps going. I’ve seen maintenance workers struggling with corroded, brittle plastics in older machines. The new blends with this additive shrink the problem—a small change with a big payoff for safety and repair costs.
Money matters in manufacturing, and so does waste. Plastics with N-Phenylmaleimide last longer, so companies don’t have to replace parts as often. That means fewer broken components end up in landfills, and fewer raw materials get pulled from the earth. Stretching product lifespan also saves energy. Researchers have studied how some blends keep their properties even after years of exposure to sunlight or harsh chemicals. Longer life cycles line up with goals of reducing resource use and keeping production costs lower. Better products at less environmental cost—who doesn't want that?
People rarely think about what keeps sockets from sparking or why car dashboards don’t turn brittle. N-Phenylmaleimide doesn’t make headlines, but its value shows up in trust—trusting that plastics and rubber will keep doing their jobs year after year. Technicians, homeowners, and engineers might not always know the name, but the benefits reach them every day. Durable parts mean fewer failures, greater safety, and reliability that holds up under real-life testing. In a world packed with electronics and synthetic materials, a molecule like N-Phenylmaleimide helps guarantee that things just work—even when no one’s looking.
N-Phenylmaleimide shows up in labs as a yellowish crystalline powder. Scoop a bit and it feels brittle, chalky. At about 89°C, the crystals start to melt. That’s a moderate melting point—just enough to set it apart from the low-melting organics, not high enough to call it stubborn. It doesn’t dissolve in water, but mix it with hot acetone or ethanol and it spreads out nicely.
Breathing around it doesn’t welcome much odor. It’s not as sharp or distinct as some of the amines and anhydrides out there. This quality makes it less likely to bother workers’ noses, but that doesn’t mean handling safety gets tossed aside. Solids like this always ask for gloves and goggles—experience has burned that lesson in well enough.
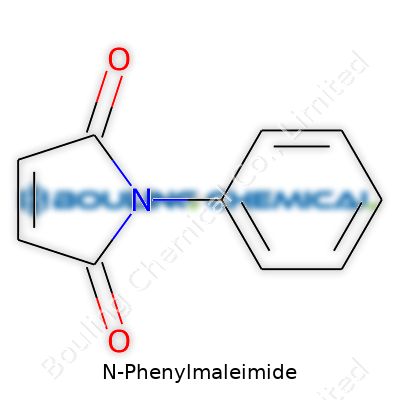
The molecule builds itself from a maleimide ring paired with a phenyl group. The maleimide bond is what a chemist might call “electron-poor”—that’s just a way to say the ring hungers for electrons and reacts with other molecules that have a surplus. This chemical hunger becomes a ticket to a broad set of uses, not just in academics but in factory floors.
N-Phenylmaleimide resists breaking down when the temperature climbs. Heating it to 200°C doesn’t make it give up easily. This thermal stability explains why manufacturers trust it in high-performance plastics and heat-resistant coatings. The ring structure, with that phenyl sidekick, stops it from falling apart below those high temperatures.
In real-world applications, N-Phenylmaleimide gets blended into copolymers, especially with styrene and acrylonitrile. The final plastics come out tougher and stiffer. Engineers working on automotive and electronics housings look for this kind of durability—cracking and warping at high temps cost money and trust. From direct experience in a research setting, adding compounds like this usually makes the final mix harder, more heat-tolerant, and less likely to melt or deform under stress.
Its chemical structure also means the compound resists yellowing from light and heat, an important advantage for manufacturers turning out parts that stay on display for years. Cheap plastics yellow and crumble; N-Phenylmaleimide additives can stretch out the shelf life with little fuss.
People making or using N-Phenylmaleimide shouldn’t toss safety data sheets aside. Like many imides, breathing in dust over time can irritate the lungs and skin. The chemical doesn’t dissolve in groundwater quickly, so spills stick around unless addressed. Workplaces keeping it on hand often use vacuum systems and strict containment, keeping the workspaces cleaner and the health risks lower.
There’s always a push to balance chemical strength with greener manufacturing. One route, drawn from my own lab days, involves using cleaner solvents during processing and seeking efficient recycling. Tight controls on waste mean less lingering contamination in soil or water.
N-Phenylmaleimide takes up a spot in labs and factories that work with polymers, adhesives, and specialty chemicals. The solid, pale yellow appearance looks harmless, but it demands respect. The safety sheet makes it clear—skin and eyes do not get along with N-Phenylmaleimide, and neither do lungs faced with its dust or fumes. Knowing this up front helps prevent mistakes that could lead to burns or respiratory trouble.
Putting this chemical on a shelf beside cleaning supplies or food just asks for problems. Segregation stands as the first line of defense. Every chemist I’ve worked with stores it in tightly sealed containers, far from sources of heat and moisture. Even small spills can create crystalline dust that finds its way everywhere. Keeping it in a dry, cool spot with limited traffic helps keep containment easy. Strong sunlight or hot pipes speed up its breakdown, so dark, room-temperature storage makes sense for the long haul.
Corrosives and strong oxidizers never mix safely with N-Phenylmaleimide, and the bottles always show this with a warning stripe or label. Flammable cabinets often come into play, though this compound itself isn’t easily ignited. Instead, the focus falls on keeping the material from mixing with incompatible substances by accident.
Anyone scooping or transferring N-Phenylmaleimide grabs gloves first—thick nitrile or neoprene, never just latex. Most labs post eye shields and respirators near the storage spot. A splash in the eye burns right away, and inhalation risks headaches or lung irritation. My early days in the lab hammered home the basics: handle inside a fume hood, never rush, and never leave the container unsealed for long. Airborne dust becomes an issue every time someone skips cleaning around the workspace.
Spills can surprise even those paying close attention. The best approach I’ve seen skips the broom and uses a vacuum with a HEPA filter meant for hazardous powders. Wet methods raise risk, since the compound reacts poorly to water and acids, sometimes releasing unpleasant vapors. Disposal follows a strict path straight to hazardous waste bins. No one sweeps a mess of N-Phenylmaleimide into the regular trash.
Many accidents happen not because people ignore labels, but because storage areas grow crowded or safety routines turn sloppy. I’ve seen labs lose research weeks from cross-contamination, all because a stray granule found its way into a neighboring shelf. Chemical burns and respiratory trouble turn up in safety records any time personal protection gets treated as optional. These costs add up fast.
Solid safety culture isn’t just about rules on a poster. Regular training, audits of storage areas, and a habit of keeping safety gear in arm’s reach set teams up for safer habits. At a plant I visited last year, tight inventory controls meant containers never stayed open or unaccounted for, and nobody hesitated to report a safety slip. The difference in accident rates from those habits told its own story.
Smart facilities track chemical use down to the gram with barcodes and digital logs. Shelves and cabinets stand labeled, and nothing shares a cubby without a second thought. Automatic door-closers and airlock entries make open-air transfer less tempting. Training sessions update everyone on hazards—especially anyone new or returning after a break.
Knowing risks, respecting boundaries, and believing in personal protection—these all shape safe storage and handling of N-Phenylmaleimide. Handled well, it drives innovation in products found all over the world; handled carelessly, it can injure and disrupt. The details count every single day.
N-Phenylmaleimide pops up in many laboratories and manufacturing floors. Manufacturers often use it to toughen plastics or improve the heat resistance of synthetic rubbers. You’d find it mixed into resins or added to paints, glues, and electronic coatings. Some chemists see it as a handy building block. It’s no mystery why this compound attracts so much industrial interest. Once you get into the technical sheets from chemical suppliers, you notice they attach hazard symbols and warning statements for a reason.
People working with N-Phenylmaleimide sometimes ignore gloves or a lab coat, thinking a small spill means nothing. What many don’t realize is how it can irritate skin or burn eyes on contact. There’s an immediate sting and redness if it splashes on unprotected skin, and the irritation can stick around. Eyes are even more sensitive. Accidental splashes can cause lasting discomfort, so chemical splash goggles aren’t just a suggestion—they’re a basic necessity.
Breathing in its dust or vapor can also bring trouble. The fine particles irritate the nose and throat, sometimes leading to coughing or headaches. Large exposures may stress the airways. Even those familiar with chemical handling can forget how much these invisible dusts can circulate in a workshop or production space.
Long-term handling without proper protection turns minor exposures into bigger health stories. Some workers develop allergies—red, itchy skin that flares up every time they’re near the product. Once someone’s skin breaks out, even small traces can keep the problem going. Chronic respiratory troubles aren’t rare either when adequate ventilation gets skipped or fume hoods stand unused.
Animal tests reported by the European Chemicals Agency show N-Phenylmaleimide’s toxic effects at high dosages, especially if it’s swallowed. It won’t cause severe acute toxicity in trace amounts, but no one in the chemical safety business recommends taking chances. Many companies label it as a “suspected” health hazard, which ought to give anyone pause who deals with bulk shipments or manufacturing lines.
Ignoring a chemical’s safety data sheet often leads to regret. I remember a colleague who skipped the gloves for a quick transfer, ended up with a stubborn rash and a real wake-up call about personal protective equipment. The risk isn’t only about splashy accidents. It’s about small lapses adding up over months or years.
Listening to credible sources, like OSHA, the CDC, or the Chemical Safety Board, isn’t bureaucracy—it’s about real lives staying healthy. Modern data point to the compound as an irritant. Its toxic effects look mild compared to some industrial chemicals, but nobody should treat that as a green light for carelessness.
Gloves, goggles, and a decent lab coat—these aren’t extras, they’re the basics that stop most short-term trouble. Proper ventilation matters too. Local exhaust fans, good airflow, and regular wipe-downs help keep dust and vapors out of breathing range. Spill kits and eye-wash stations shouldn’t gather dust in the corner.
Many workplaces benefit by reviewing their protocols with regular safety briefings. Marking containers clearly, locking away raw chemicals after hours, and keeping up on training turn a hazardous material into a controlled one. Chemistry will never be risk-free, but respect and routine beat bravado every time.
In the fine chemicals world, regular folks might not think much about N-Phenylmaleimide, but people making plastics, resins, or specialty rubbers care about this stuff a lot. Most of the batches coming out of established manufacturers clock in at or above 99% purity. A lot of companies push for this number because going lower runs the risk of extra byproducts getting in the way, and if those invade a polymerization process, things can fall apart in a hurry. Raw material glitches in the end show up as failed quality tests and warranty claims.
Publishing consistently at that high purity isn't just a claim tossed onto a spec sheet. Makers routinely lean on standard gas chromatography and HPLC checks, so they can spot traces of side-products and moisture. Labs chase that low ppm (parts per million) trace of impurity, since even a little hitch in purity can trigger yellowing, lower strength, or batch rejections down the supply chain. Speaking as someone who’s watched a production line grind to a halt thanks to subpar raw materials, these extra decimal points in spec sheets aren’t marketing fluff—they’re insurance for everyone in the pipeline.
Most customers order in kilogram-sized portions. Small research labs tend to like 100 g bottles or 500 g flasks, usually vacuum-sealed and stored in cool, dark places. Larger buyers load up drums, mostly 25 kg fiber or steel drums lined with polyethylene bags that zip tight and keep humidity out. I have seen shipping forms laying out pretty clearly: keep it away from heat, moisture, and sunlight, because this powder gets clumpy if handled wrong.
I’ve had to crack open containers of N-Phenylmaleimide more than once, and anyone working with it knows you want it bone dry. Even a little dampness creeping through during storage can push moisture levels into double digits, and then no one’s happy—clumping, poor flow, and the headache of reprocessing. Given that, a lot of places throw in desiccant packs or use double-bagged liners.
Now and then, smaller operators might skip a few steps, but serious chemical suppliers know buyers will walk if the product pops open lumpy or tainted. Regulatory bodies in major markets, like the EU or US, sniff out supply chains quickly. If the purity slips below 98.5% or the packaging doesn't hold up to shipping, risk follows. Recalls or rejections eat back more than any savings from cutting corners.
Global transportation adds another layer. Fluctuating temperatures and humidity in shipment from Asia or Europe take a toll on this compound, and I’ve seen more than a few distressed calls when drums land out-of-spec. Reliable tracking, temperature controls, and full batch records matter just as much as what’s inside the container. Smart buyers ask for the latest certificate of analysis tied to their lot number, and solid suppliers provide them as a standard practice.
A little extra attention to purity pays dividends down the road—not just in the lab, but in the reputation of everyone handling N-Phenylmaleimide, from supplier to end user. Investing time in quality packaging and careful logistics keeps the headaches at bay and lets each link in the chain focus on what they really want: a product that performs every single time.
| Names | |
| Preferred IUPAC name | 1-phenyl-1H-pyrrole-2,5-dione |
| Other names |
N-Phenylmaleimide N-Phenyl-2,5-pyrroledione N-Phenyl-2,5-dioxopyrrolidine N-Phenylmaleamic anhydride N-Phenylmaleimid |
| Pronunciation | /ɛnˈfɛn.ɪl.məˈleɪ.ɪ.maɪd/ |
| Identifiers | |
| CAS Number | 941-69-5 |
| Beilstein Reference | 1206073 |
| ChEBI | CHEBI:51451 |
| ChEMBL | CHEMBL107187 |
| ChemSpider | 14399 |
| DrugBank | DB08746 |
| ECHA InfoCard | 100.065.900 |
| EC Number | EC 207-838-8 |
| Gmelin Reference | 8219 |
| KEGG | C06585 |
| MeSH | D016207 |
| PubChem CID | 8373 |
| RTECS number | TR6825000 |
| UNII | 38FSB6L6GH |
| UN number | UN3077 |
| Properties | |
| Chemical formula | C10H7NO2 |
| Molar mass | 173.17 g/mol |
| Appearance | light yellow crystalline powder |
| Odor | Odorless |
| Density | 1.22 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 0.96 |
| Vapor pressure | 0.000227 mmHg at 25°C |
| Acidity (pKa) | 7.2 |
| Basicity (pKb) | 7.68 |
| Magnetic susceptibility (χ) | -62.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.625 |
| Viscosity | 3.4 mPa·s (25 °C) |
| Dipole moment | 2.61 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 332.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -42.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3221 kJ/mol |
| Pharmacology | |
| ATC code | |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and eye irritation, may cause respiratory irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS07, GHS09 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P264, P280, P302+P352, P305+P351+P338, P332+P313, P337+P313, P362+P364 |
| NFPA 704 (fire diamond) | 2-1-2-Ξ |
| Flash point | Flash point: 196°C |
| Autoignition temperature | 575°C |
| Lethal dose or concentration | LD50 oral rat 700 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral-rat LD50: 640 mg/kg |
| NIOSH | SN9000000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0-40°C |
| IDLH (Immediate danger) | IDLH: Not established |
| Related compounds | |
| Related compounds |
Maleimide N-Ethylmaleimide N-Methylmaleimide Phthalimide |