N,N,4-Trimethylpiperazine-1-ethylamine brings together a fascinating background that traces back to the wave of synthetic amines in the late twentieth century. Chemists began with the piperazine core over a century ago due to its straightforward structure and versatility, but the surge in custom amine production in pharmaceutical and materials science fields put derivatives like this one on the map. Multi-substituted piperazines like N,N,4-Trimethylpiperazine-1-ethylamine grew in popularity as researchers understood how tweaks in the molecule could dramatically change properties. Looking at the chemical landscape, major pharmaceutical milestones — from new antihistamines to enzyme inhibitors — often featured these specialized scaffolds. The ethylamine group and carefully placed methyls offered a new balance in basicity and solubility, giving an edge in the search for next-generation modulators and solvents.
The compound presents as a clear or lightly colored liquid, sometimes with a faint amine odor noticeable in lab settings. With the backbone of piperazine and the additional functional groups, it allows for structural flexibility many researchers chase. The combination of amine nitrogens and three methyl groups separated by an ethyl chain lets it interact differently with acids, bases, and organic solvents compared to simpler piperazines. Suppliers typically distribute it in high-purity grades, suitable for both industrial and academic research. Because of regulatory scrutiny around some piperazine derivatives due to their psychoactive potential, this variant’s label often includes dedicated handling guidelines. Over the years, product availability has remained steady, with a modest but reliable demand from medicinal chemistry and specialty chemical sectors.
This compound appears as a viscous, colorless liquid under normal conditions, with moderate volatility and strong affinity for polar solvents. Its boiling point sits above 180°C, and the melting point stays well below room temperature, making it easy to handle as a liquid across most climates. The density hovers just below that of water, and it dissolves readily in ethanol, methanol, and dichloromethane. Its basicity reflects typical piperazine chemistry, but the extra methyls depress the pKa ever so slightly, resulting in a molecule with lower reactivity toward certain acids. It tends not to form crystalline hydrates, so storage rarely sees any surprising precipitation or clumping.
Technical documentation often lists the compound with purity greater than 98%, water content below 1%, and minimal side-products. Standard GC-MS and HPLC assays confirm the chemical identity and rule out related impurities. Commercial labels call for storage in tightly sealed glass or HDPE containers at temperatures between 2°C and 8°C, away from sunlight and strong oxidizers. Certification often includes lot-specific traceability and compliance with EU REACH and US TSCA regulations. Any company selling to pharmaceutical labs or regulated industries must include an unambiguous SDS sheet covering protective equipment, spill management, and disposal. Labels stick to a hazard classification system, clearly identifying the risks related to skin, eyes, and vapor inhalation.
Synthesis involves N-alkylation reactions on a piperazine backbone. Chemists often begin with 1-ethylpiperazine, reacting it with methyl iodide under phase transfer conditions, using a base such as potassium carbonate to drive methylation. Sometimes, the sequence flips, starting with N,N-dimethylpiperazine and using a two-step sequence to introduce the ethyl group on the nitrogen. Post-reaction work-up typically uses liquid-liquid extraction, followed by vacuum distillation and column chromatography for purification. Careful temperature control and dry atmospheres prevent side reactions, keeping the final product consistent batch after batch.
This molecule reacts predictably as a secondary and tertiary amine. Primary pathways include acylation of the nitrogen with acid chlorides or anhydrides, yielding substituted amides, which often surface in drug discovery work. Reduction and oxidation steps allow for functional variation, especially if researchers want to tune solubility, basicity, or binding affinity. More complex chemistry sometimes builds on the ethylamine side chain, attaching sulfonates, phosphates, or alkyl groups for tailored interactions with biomolecules. Skirting oxidative degradation and hydrolysis keeps the product stable during synthetic workups, minimizing waste and unwanted byproducts.
Several different commercial suppliers assign alternate names, including 1-Ethyl-N,N,4-trimethylpiperazine, 4-Methyl-1-ethyl-N,N-dimethylpiperazine, and Ethyl(4-methyl-1-piperazinyl)dimethylamine. Database registration numbers span CAS, EC, and InChIKey codes, aligning it with global regulations and research standards. Industry catalogs feature short names like “TMP-EA,” which simplifies ordering and documentation. Accurate nomenclature matters for procurement and regulatory compliance, since small variations in naming can easily lead to confusion about chemical identity or application scope.
Working with any substituted piperazine, safety teams focus on inhalation, dermal, and ocular risks. In my own lab experience, gloves, goggles, and open fume hoods made a practical difference between a routine day and irritated skin or headaches from vapor exposure. The product’s volatility, while lower than some amines, can still leave a lingering presence, especially during weighing or dilution. Emergency wash protocols don't just offer peace of mind—they show up in every oversight review. Proper labeling keeps everyone clear on what’s inside that oddly-shaped bottle or amber jar. Compliance officers regularly monitor handling logs, disposal manifests, and local air quality standards. Regulatory audits might not be every chemist’s favorite, but that structure gives confidence to everyone in the room.
Researchers and manufacturers value this compound for how it slots into pharmaceutical synthesis, agrochemical research, and specialty solvent development. Medicinal chemists tweak the scaffold trying to modulate activity for new CNS agents, enzyme inhibitors, or receptor modulators. Specialty coatings sometimes blend it for anti-static or anti-corrosive properties. Custom polymer developers see opportunity to alter solubility profiles in novel plasticizers. Drug discovery teams look for analogs that sidestep patent thickets or deliver unexpected secondary properties. Universities and startup incubators keep it in small batches for exploratory projects that test new hypotheses in both wet- and dry-lab environments.
Research teams keep exploring what the combination of the piperazine ring, methyls, and ethylamine tail can do in more complicated molecules. Academic groups and startups use it as a launching point for combinatorial chemistry, popular in building compound libraries for high-throughput screening. Projects funded by major pharmaceutical companies often chase target engagement and subtype selectivity, running SAR (structure-activity relationship) studies that rely on subtle changes to the amine’s structure—changes N,N,4-Trimethylpiperazine-1-ethylamine makes possible. In my interactions with chemists seeking new methods, this compound gets picked out regularly for toolbox expansion because it balances basicity with manageable reactivity and solid pharmacokinetic attributes.
The academic literature on toxicity covers both direct exposure studies and metabolism predictions. Standard cell-line assays show moderate cytotoxicity at high concentrations, not wildly different from other multi-substituted piperazines. Small animal tests demonstrate low acute toxicity, but repeated exposure over weeks raises liver enzyme activity in rodents, hinting at cumulative effects with prolonged dosing. Reports of skin sensitization and mild respiratory irritation cropped up in chemical handling studies from several university labs. Metabolite screening in simulated human liver microsomes maps out N-dealkylation as a primary clearance pathway, generally leading to less toxic breakdown products. Safety data remains incomplete for long-term exposure, reinforcing a laboratory culture focused on protective equipment, careful measurement, and rigorous reporting.
The landscape for N,N,4-Trimethylpiperazine-1-ethylamine keeps evolving as new applications surface. Synthetic biologists and chemists drive interest by using it as a starting block for compounds that nudge harder-to-reach biological targets. The shift toward precision medicine keeps companies invested in refining related structures, aiming for products with better selectivity and fewer side effects. Regulatory changes sometimes push researchers to find derivatives that skirt legal restrictions; here, the subtlety of amino substitution provides crucial room to maneuver. Automation and AI-guided synthesis are starting to touch even legacy molecules—this one included—by streamlining optimization cycles and bringing better yields and new analogs inside a few weeks. With investment in sustainable chemistry building momentum, greener routes for production and purification come under more scrutiny. Trying to close the loop between discovery and compliance never stops chemists from seeking safer, more effective compounds.
N,N,4-Trimethylpiperazine-1-ethylamine isn’t one of those compounds people hear about at the lunch table. The name itself usually only echoes down the halls of chemical companies, research labs, and sometimes pharmaceutical workshops. But behind the long scientific label sits a molecule that quietly supports several practical fields, especially organic synthesis and drug development. Anyone who’s tried to synthesize complex molecules or tinker with chemistry knows how value often shows up in unexpected forms.
If you look through the methods pages of scientific papers or talk to seasoned chemists, you'll notice this compound often pops up during the creation of heavy-duty organic structures. Its main utility comes from its two functional groups: an amine and a methyl-substituted piperazine ring. These rings and chains act like building blocks. Chemists use them as intermediates to link bigger molecules, chase new medicines, or even create specialized materials for technology applications.
The pharmaceutical sector especially places N,N,4-trimethylpiperazine-1-ethylamine on a short list for research, since piperazine cores show up in many drugs. The anti-allergy drug cetirizine, for instance, and some anti-psychotic compounds, rely on similar backbone structures. With chemical modifications enabled by this compound, there’s the potential to fine-tune drug candidates for better performance or fewer side effects. It’s not just academic—incremental tweaks over the years have improved everything from antidepressants to cancer treatments. This is not the kind of ingredient you see on your medicine label at home, but it sits under the hood, powering innovation from behind the scenes.
Few people outside chemistry ever hear about compounds like this unless a headline about drug shortages or pharmaceutical pricing pops up. Still, without these basic building blocks, we lose the chance to develop new treatments or improve safety protocols. I remember working alongside researchers who spent days just trying to coax a stubborn molecule to react. Sometimes all it took was the right intermediate, and progress sprang back to life. It showed me firsthand how basic research tools can open the door to real-world solutions.
Access to specialty chemicals like N,N,4-trimethylpiperazine-1-ethylamine isn’t always straightforward. Labs in low-resource settings or smaller universities often face supply chain snags or cost issues. That tips the balance in favor of big corporate centers, slowing global progress. Open-source chemical databases and cost-sharing initiatives might help level the field, letting more researchers try their hand at discovery. Policy approaches can tackle export or import hurdles, especially for promising countries that want a bigger say in pharmaceuticals.
Environmental impact demands more thought, too. Chemical production sometimes leaves harmful residues, so green chemistry practices matter. Encouraging the industry to make these compounds cleaner or find recycling paths could cut down waste and lessen hazards for workers. I’ve spoken with manufacturers who are starting to invest in solvent-free reactions and renewable starting materials—that feels like a step in the right direction.
Put simply, niche chemicals drive innovation by setting up a chain of discoveries. N,N,4-trimethylpiperazine-1-ethylamine plays its part as a modular element for researchers and developers, quietly linking the scientific workbench to tomorrow’s breakthroughs. Keeping the supply open, safe, and efficient helps the whole field grow, pushing the pace toward new ideas in medicine, materials, and beyond.
Few people outside a lab recognize the name N,N,4-Trimethylpiperazine-1-Ethylamine, but this chemical takes its place in real research and industrial projects. Years around labs have taught me the only right way to treat compounds with complex names: Respect them. This compound causes irritation, and its vapors aren’t friendly to eyes, skin, or lungs. Mistakes happen fast. Once, I watched a colleague splash a pipette tip—nothing tragic, but it meant hours flushing eyes, scaring everyone in the room. That image beats any training video.
Gloves, safety glasses, and a solid lab coat don’t make anyone invincible, but they make accidents much less scary. Splashes sneak past a thin glove or sleeve. Chemical goggles with side shields always seemed overkill in college—until the first time I saw a splash leap sideways past basic safety glasses. Nitrile gloves stand between a minor mishap and a trip to the ER.
Good shoes matter, too. Closed-toe shoes save a lot of grief if a spill hits the floor. I've seen sandals and experiments in the same room. That never turns out well.
Working with N,N,4-Trimethylpiperazine-1-Ethylamine calls for a real fume hood. On a warm day, some folks think a cracked window works fine, but vapors travel. Pulling air away with a certified hood lowers exposure, plain and simple. One whiff is sometimes all it takes for a burning throat or tired eyes to set in. Labs that keep hoods well-maintained avoid those problems.
Chemicals left in unlabeled bottles cause panic and confusion. Legible labels with the full name, concentration, and a date keep everyone on the same page. In my early days, I saved a friend from nearly mixing two clear liquids stored side by side, all because we kept strict labels. Shelving matters too—this compound belongs away from acids, oxidizers, or any kind of food storage.
Lockable cabinets add a layer of security. Quick access bottles can never sit near eye level or inside crowded spaces with flammable solvents.
Sinks don’t cut it. Emergency gear cuts down response time after a spill or splash. I once relied on a communal sink out of habit, waiting seconds that felt like an eternity. After that, I pushed for better station placement at every workplace. A dedicated eye wash becomes invaluable during crisis moments, especially when dealing with volatile or irritating substances.
Spill kits stocked with absorbent pads, neutralizers, and disposable gloves stay ready for anything. Clean-up shouldn’t start with a paper towel or water. Every second counts, and preparation keeps the mess contained before it spreads.
Written guidelines feel dull until an accident hits home. Training sessions, run-throughs of spill drills, and honest conversations keep everyone alert. Peer reminders about gear and practices double as life-saving habits, not nagging. In the end, each precaution isn’t just a rule—it’s a step toward getting everyone home safe at the end of the day.
Some chemicals can seem like a mouthful until you break them down piece by piece. N,N,4-Trimethylpiperazine-1-ethylamine falls into that category. Its name might sound intimidating, but digging deeper always helps. This molecule takes the familiar six-membered piperazine ring—a favorite in pharmaceutical chemistry—and decorates it with three methyl groups and an ethylamine chain. Chemists write its chemical formula as C9H21N3. Every letter and number packs meaning. Nine carbons build most of the structure's backbone, supporting twenty-one hydrogens and three nitrogens. The arrangement isn’t arbitrary—each atom brings its own chemistry to the party and shapes how this molecule acts when it gets mixed with others.
The molecular weight of a compound tells you much more than just how heavy a molecule is. It guides dosing, solubility, transportation within the body, and even how a compound might get filtered in a production process. For N,N,4-Trimethylpiperazine-1-ethylamine, the molecular weight clocks in at about 171.29 g/mol. That number comes from a simple calculation: carbons (12.01 x 9), hydrogens (1.008 x 21), nitrogens (14.01 x 3). Scientists don’t memorize all these digits for fun; accuracy in weights underpins just about every test, recipe, and synthesis step in modern chemistry.
Compounds like N,N,4-Trimethylpiperazine-1-ethylamine don’t live in a vacuum. They show up in the world, popping up as building blocks for pharmaceuticals or specialty chemicals. In my work with small-scale synthesis, versatility can spell the difference between failure and a breakthrough. A piperazine backbone supports active drugs from antihistamines to anti-tumor agents. The methyl and ethyl groups in this case aren't picked for looks—they fine-tune how the molecule behaves, slips through membranes, or holds its shape in a binding pocket.
But chemical benefits bring chemical responsibilities. These tertiary and quaternary amines sometimes introduce challenges: increased basicity, volatility, and often a push-pull between water and fat-loving properties. Working with molecules like this, I’ve learned to keep gloves on and my ventilation checked. Safety guidelines, material safety data sheets, and proper storage never go out of style. The risks of exposure, even for low-toxicity compounds, can add up over time.
Echoing many colleagues, I believe the best solution involves combining practical lab experience with careful documentation. Before running a reaction or handling bulk material, a chemist needs access to full, clear safety profiles. Training on hazards, exposure controls, disposal methods, and potential environmental impact must be standard practice. Chemical suppliers could help by providing more granular and up-to-date safety and regulatory information right alongside the purchase.
Innovation in chemistry depends as much on safe stewardship as it does on clever design. For N,N,4-Trimethylpiperazine-1-ethylamine, knowing the numbers—chemical formula, molecular weight—serves as the starting line rather than the finish. The rest depends on skill, respect for the risks, and sharing knowledge between scientists, engineers, and safety professionals.
In any lab or warehouse setting, chemicals like N,N,4-Trimethylpiperazine-1-ethylamine don't give much warning before problems start. I’ve spent years moving through labs, and the level of care given to chemical storage can often make or break not only the results of an experiment, but also the safety record of a workplace.
Let’s talk about what this compound can do: it’s an aliphatic amine, which means it brings flammability and reactivity to the table. Any amine leans toward volatility and the risk of fumes, and even one spill can cause headaches—literally and figuratively. Exposure raises the chances of irritation to the skin, eyes, and airways. For folks handling it every day, repeated contact usually leads to trouble if precautions slip. Oversight on the shelf life or labeling multiplies the risk, too.
Storing this compound safely takes more than tucking it on a back shelf. From my experience, the smartest practice always keeps it away from heat and direct sunlight. Think cool, dark cabinets, with solid ventilation. You want the temperature to stay steady—chemical stability drops fast with swings. Moisture is no friend either, so always leave it inside tight, properly labeled containers. I’ve seen old jars with rubber stoppers crack or warp, and that sort of neglect can cause vapors to get out, contaminating the area or the next experiment.
Flammable amines need to stay far from acids, oxidizers, and anything that could spark a reaction. Mixing storage can cause a dangerous cocktail—separating incompatible chemicals is one habit that saves trouble in the long run. Trained staff should sort and double-check labels, making sure nobody rushes through shelving after a delivery. Accidents often start with fatigue or distraction at moments like these.
Record-keeping isn’t glamorous, but it sure proves valuable in a crisis. Log entries for who accessed the chemical and when, combined with regular inventory checks, help spot leaks before they grow into incidents. Outdated or cracked containers need prompt disposal, and a simple checklist catches mistakes early.
Any storage area should display clear signage, alarm systems, and keep spill kits nearby. Training each new staff member matters as much as having the equipment itself. One experience with a poorly handled chemical splash taught me that quick access to eyewash stations and showers sometimes means the difference between an inconvenience and a medical emergency.
Building a good safety culture doesn’t happen by accident. Managers who set the tone through routine inspections, surprise drills, and honest conversations about near-misses inspire everyone to keep a careful eye on storage. All the best containers and cabinets in the world won’t protect anyone if they’re used carelessly or overloaded.
Institutions that value safety often invest in modern ventilation and fire protection. Proper airflow cuts down on the inhalation risk during spills. Automatic fire suppression can limit a disaster before staff even react.
Safe storage requires more than a manual or set of rules; it depends on keeping people engaged and alert. Sharing stories from the field, reviewing the latest updates on chemical safety, and promoting responsibility helps keep dangerous shortcuts out of everyday habits.
N,N,4-Trimethylpiperazine-1-Ethylamine isn’t a chemical most folks toss around at a backyard BBQ, but it plays a niche role in the labs of researchers and specialty manufacturers. The subject of purity, in my experience, draws more debate than almost any single characteristic on a chemical’s certificate of analysis. Different grades or purities tell a lot not just about where the compound comes from, but mainly about what people plan to do with it. For a chemist aiming to run sensitive pharmaceutical synthesis, the tiniest amount of contamination can unravel a whole project. A production engineer in specialty coatings or high-performance plastics might accept a wider impurity profile if it keeps the process moving and the cost down.
One lesson I’ve learned from years spent wandering chemical storerooms and working next to fume hoods: never assume that one bottle matches the next. Some suppliers tag this compound as “analytical grade,” giving users the green light for precise applications. These high-purity stocks usually land above 98 or even 99 percent. Higher purity means less cleanup on the back end, less troubleshooting, and a better chance of avoiding costly surprises in the final product. It sounds simple, but often, mistakes happen right at the purchasing step, because two bottles with the same label might carry invisible risks inside.
Technical or industrial grades do exist, usually at lower price points. These may hold to looser standards—90 to 95 percent purity isn’t rare. Such grades might work just fine in less sensitive settings: think batch manufacturing where the leftover trace compounds don’t interfere with the product’s job. But these impurities can create headaches if they piggyback into formulations headed for tightly regulated uses—like pharmaceuticals or medical devices. Several cases come to mind where a supposedly minor contaminant threw off an entire analysis, or even pawned off minor health risks if left unchecked.
Regulatory authorities like the FDA or European Medicines Agency don’t leave much to chance. Their focus falls hard on trace contaminants, even at parts-per-million. People spend thousands on validation tests, chromatography runs, and even DNA fingerprinting of raw materials. Documentation trails need more than a supplier’s word. Beyond that, reputable suppliers often provide detailed certificates of analysis, including heavy metals, water content, and even residual solvents. Some go further with ISO or GMP certifications that guarantee tighter oversight across every stage of handling and shipping. My own preference? Always ask for recent, third-party analysis before pulling the trigger on an order—especially for new sources or new lots.
Lower purities can slide by in petrochemical blending or preliminary research, but higher grades dominate where any direct human or animal contact is involved. Also, some impurities don’t just ruin a product—they can trigger fires or unplanned reactions, especially if the user isn’t aware. Good storage and prompt handling help prevent dangerous surprises. If you ever get a bottle without clear labeling or with a missing safety data sheet, treat it as a red flag.
N,N,4-Trimethylpiperazine-1-Ethylamine comes in more than one grade, and not every application calls for the same level of purity. Knowing what’s inside a drum matters just as much as where it’s going. Careful documentation, regular supplier audits, and in-house testing save both money and headaches down the road. One size never fits all in chemistry, and anyone handling specialty compounds should keep that lesson close.
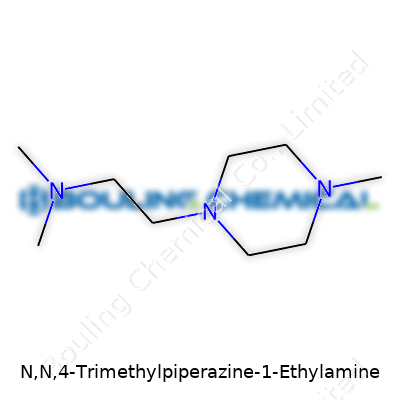
| Names | |
| Preferred IUPAC name | 1-Ethyl-N,N,4-trimethylpiperazin-1-amine |
| Other names |
N,N,4-Trimethyl-1-(2-aminoethyl)piperazine 1-(2-Aminoethyl)-N,N,4-trimethylpiperazine N,N,4-Trimethyl-1-piperazineethanamine |
| Pronunciation | /ɛn ɛn fɔːr traɪˈmɛθɪl paɪpəˌreɪziːn wʌn ˈɛθɪləmiːn/ |
| Identifiers | |
| CAS Number | 773878-70-9 |
| 3D model (JSmol) | CCN1CCN(C)CC1C |
| Beilstein Reference | 1718739 |
| ChEBI | CHEBI:187658 |
| ChEMBL | CHEMBL2521818 |
| ChemSpider | 7040159 |
| DrugBank | DB08373 |
| ECHA InfoCard | 15d16a13-e33e-4708-b942-fefc72a574b9 |
| EC Number | EC 620-540-9 |
| Gmelin Reference | 142246 |
| KEGG | C18745 |
| MeSH | D010921 |
| PubChem CID | 16194228 |
| RTECS number | UF9555000 |
| UNII | Z4VRN90N3N |
| UN number | UN3276 |
| Properties | |
| Chemical formula | C9H22N2 |
| Molar mass | 186.306 g/mol |
| Appearance | Colorless to light yellow liquid |
| Odor | Amine-like |
| Density | 0.89 g/cm3 |
| Solubility in water | Soluble |
| log P | 0.57 |
| Vapor pressure | 0.292 mmHg at 25°C |
| Acidity (pKa) | pKa ≈ 9.8 |
| Basicity (pKb) | 4.32 |
| Magnetic susceptibility (χ) | Magnetic susceptibility (χ) of N,N,4-Trimethylpiperazine-1-Ethylamine: -74.5×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.490 |
| Viscosity | 0.89 cP (25 °C) |
| Dipole moment | 2.94 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 355.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -67.4 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4848 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes severe skin burns and eye damage. Causes serious eye damage. Harmful if inhaled. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302 + H312 + H332: Harmful if swallowed, in contact with skin or if inhaled. |
| Precautionary statements | P261, P264, P271, P272, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P333+P313, P337+P313, P362+P364, P363, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) | 1-3-0 |
| Flash point | 116.9±25.6 °C |
| PEL (Permissible) | Not established |
| REL (Recommended) | 100 mg |
| Related compounds | |
| Related compounds |
N-Methylpiperazine N,N-Dimethylpiperazine N-Ethylpiperazine 1-Ethylpiperazine 1,4-Dimethylpiperazine N,N,4-Trimethylpiperazine 4-Methylpiperazine |