Chemists chasing stable amine compounds in the mid-twentieth century found themselves intrigued by the unique durability of hindered amines. Among those, N,N,2,2,6,6-Hexamethyl-4-piperidinamine stood out. It rose to prominence as researchers probed ways to extend the life of plastics and coatings. By the 1970s and 80s, industry insiders recognized its molecular structure—six methyl groups guarding the piperidine nitrogen—as a breakthrough. Its design stops oxidative attack, unlike ordinary amines that falter under UV abuse. Old chemical catalogs rarely mentioned such bulky amines. Now, it's on shortlists across the specialty chemicals sector, showing how insight fuels leapfrogging progress in product engineering.
N,N,2,2,6,6-Hexamethyl-4-piperidinamine attracts attention wherever light or oxygen threaten lifespan. This molecule lives in the backbone of many high-performance stabilizers. Chemists favor its ability to lend extraordinary protection with very little additive needed. Compared to earlier options, this amine gives more control, less yellowing, and a longer window before polymers break down. I’ve seen polymer labs treat it like gold dust, calculating loadings down to tenths of a percent and still getting years of improved performance. Manufacturers favor it for its punchy results with minimal interference in other material characteristics.
This compound arrives as a white crystalline solid or colorless liquid—those who have handled it will remember the vaguely ammoniacal, sharp smell. With a melting point hovering near 35°C, it easily liquefies during summer processing or with gentle heating. Solubility spans many organic solvents, from low-boiling ethers up to tough aromatics and alcohols. Its robust tertiary amine function shrugs off oxidizers that would tear apart weaker cousins. Provided you store it away from acids and moisture, shelf life tracks in years, not months.
Suppliers measure purity by gas chromatography, aiming above 98% for specialty and pharmaceutical applications. Water content gets kept under 0.5%; residual solvents, even lower. UN hazard labels warn of irritation—packing groups may change depending on jurisdiction. Labeling includes the IUPAC name, various synonyms, batch number, net weight, and a date of manufacture. Proper labeling reflects the global paths this chemical often travels, traded between American, European, and Asian markets under strict regulatory frameworks.
The classic synthesis begins with 2,2,6,6-tetramethylpiperidine as a precursor, often prepared from piperidine via exhaustive methylation. This process typically uses excess methyl iodide and base, managed in large vessels with tight pressure controls. Following that, the secondary amine converts to its hexamethylated derivative using further methylating agents—often formaldehyde and formic acid under Eschweiler-Clarke conditions, or simple alkyl halides, depending on scale and waste control preferences. The whole process highlights a recurring theme: the chemical industry’s need to juggle cost, purity, and waste, rather than just pouring in reactants and hoping for the best.
N,N,2,2,6,6-Hexamethyl-4-piperidinamine resists many attacks, thanks to its crowded structure. It doesn’t quench acids easily, which lets it ride through polycondensation or polymerization steps without breaking down. If you’re after derivatives, alkylation or acylation handles do exist, although getting past those methyl “bodyguards” around the nitrogen takes a forceful touch or precise catalysis. This reluctance to react proves crucial for end-users—nobody wants runaway side reactions turning pricey stabilizer into colored byproducts that accelerate damage instead of stopping it.
Trade literature and safety sheets list several aliases: it crops up as HMPA, hexamethylpiperidinamine, and on rare occasions as 4-aminotetramethylpiperidine. Companies blend this amine into complex stabilizer packages, often rebadging under various tradenames. Yet, the piperidine core and double-triple methyl caging always give it away to anyone in the know. Knowing these synonyms helps avoid confusion in procurement, logistics, and regulatory checks. More than once, I’ve seen a project delayed because a shipment under one name wasn’t linked to its alternate label in customs paperwork.
Workers handle N,N,2,2,6,6-Hexamethyl-4-piperidinamine with gloves, goggles, and well-ventilated benches. Contact brings risk of skin, eye, and airway irritation. Facilities invest in local exhaust ventilation and chemical-resistant work surfaces to keep exposure below regulatory limits. Spilled material gets contained and neutralized, with waste containers labelled and tracked. Training drills emphasize the importance of material isolation from acids and oxidizers. Fire control plans treat it as a combustible, so no sparks or open flames in the blending area. Companies adhering to strict operational standards see improved worker health and fewer shutdowns for decontamination.
You’ll find N,N,2,2,6,6-Hexamethyl-4-piperidinamine most often in light and thermal stabilizer blends for plastics, automotive coatings, and high-end aerospace composites. The electronics sector uses it in conformal coatings to keep printed circuits flexible and clear after years of heat cycling. Synthetic fiber plants lean on it to protect polypropylene and polyolefin filaments in outdoor applications—to me, that’s a lifesaver for anything touching the agricultural or construction markets. Paint chemists bank on its presence to keep building facades looking fresh rather than chalked and patchy. The growing market for UV-cured 3D printing resins and high-strength adhesives relies more on this molecule every year, pushing its boundaries further.
Research groups dig deep into structure-property relationships, testing analogues with different (sometimes bulkier) alkyl arms or tweaking the ring for subtle effects on volatility and compatibility. Academic collaborators, supported by industry consortia, routinely measure performance against new environmental standards—especially as outdoor durability standards shift. In the lab, chemists chase improved purity with less toxic precursors, motivated by regulatory pressure and real-world exposure data. Open-access journals feature new derivatives for specialty uses, like encapsulated forms or “smart” stabilizers that activate under specific conditions. Tech transfer between fundamental research and scalable production represents the major challenge and promise here.
Toxicologists have scrutinized N,N,2,2,6,6-Hexamethyl-4-piperidinamine for its effect on humans and wildlife. Standard animal models point to moderate toxicity for oral and dermal routes, which justifies all those safety measures on the factory floor. Chronic exposure studies highlight mild to moderate organ impacts at high doses, and environmental teams keep a close eye on runoff risks—its high stability means it can persist in water without breaking down. Concerns about skin sensitization and inhalation hazards led regulatory bodies to include it on chemical watchlists. Scientists continue to chase greener alternatives or truly biodegradable variants, while regulatory filings shift as new international guidelines emerge.
Growth in outdoor consumer goods, renewable energy installations, and next-gen 3D manufacturing fuels new demand for robust UV stabilizers. This molecule’s underlying chemistry remains a gold standard for toughness, even as labs keep tweaking it for better safety and lower environmental persistence. I see the near future focused on designing blends that hit tougher safety limits while cutting production footprints. Regulations may tilt investment toward more reusable forms, or composite packaging that limits migration into sensitive environments. Data-driven additive selection, shared between end users and suppliers, could speed up the rollout of safer, more efficient stabilizer systems. The call for greener chemistry and clean-label products will keep challenging the community to evolve this workhorse compound without giving up on its unmatched endurance.
N,N,2,2,6,6-Hexamethyl-4-piperidinamine pops up in unexpected places. You’ll find it in products that touch our lives daily—paints, plastics, coatings, and even some crop protection. This chemical works as a light stabilizer, almost like sunscreen for materials. Without molecules like this, sunlight can break down plastics faster, leave outdoor furniture looking faded and brittle, and ruin car interiors in just a season or two. You might not think much about the clear coat on your car or the gloss on a lawn chair, yet both rely on additives like this amine for durability in sunlight.
Dealing with plastics at work opened my eyes to how much stabilizers shape product quality. A manufacturer can save costs upfront by skipping the right stabilizer, but that means returns, unhappy customers, and trust lost down the road. Facts show light, heat, and oxygen all break down plastics through a process called photo-oxidation. Papers from chemical safety journals give clear evidence: using a hindered amine like N,N,2,2,6,6-hexamethyl-4-piperidinamine blocks that cycle, slows aging, and extends the lifespan of coatings and plastics. Data from the coatings industry points to an increase of up to 300% in outdoor durability with these additives.
Every chemical brings risks. The European Chemicals Agency lists N,N,2,2,6,6-Hexamethyl-4-piperidinamine as hazardous in concentrated form, warning about inhalation and skin contact. Experience in a factory with loose controls made me value good personal protective gear—gloves, goggles, vented hoods. Chronic exposure may irritate lungs and skin. If this chemical spills, it reaches water, and that can harm aquatic creatures. The right approach means strong containment, good engineering, clear hazard training, and waste management based on guiding documents from OSHA or EPA. Repeated health checks and environmental testing protect workers and the environment.
Relying on additives creates long-life products, which means less landfill waste and more value over time. Some manufacturers have started searching for bio-based light stabilizers, but right now, many depend on synthetic amines for performance. Researchers suggest recycling plastics can sometimes carry residual stabilizers forward, giving reused materials a fighting chance outdoors. In my experience, pushing for greener chemistry usually starts with talking to suppliers and sharing trial results with customers who ask tough questions about product safety and transparency.
Government regulation sets the rules, but companies and consumers both hold real power. If you’re a consumer, ask about the coating on your outdoor gear. Read the safety data sheet if you work with plastics or paints. If you manage a facility, encourage a safety culture. By sharing what you find—reliable studies, clear product labeling, even honest talk about risks—the industry shifts toward smarter, safer choices. N,N,2,2,6,6-Hexamethyl-4-piperidinamine won’t make headlines, yet the choices around it directly affect things we use and rely on every day.
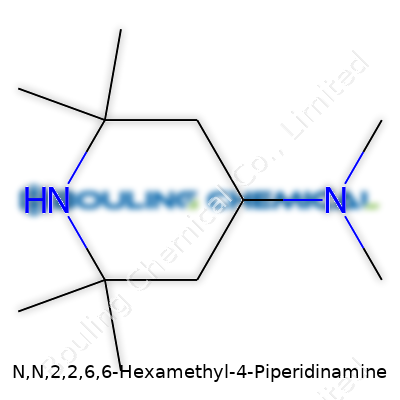
Reading about N,N,2,2,6,6-Hexamethyl-4-Piperidinamine takes me back to afternoons hunched over chemistry textbooks, sketching out molecules while the scent of solvent stuck to the air. This mouthful of a compound name actually lays out the structure clearly for anyone who digs through organic chemistry’s logic. It’s a piperidine core clogged with six methyl groups, and an extra amine on the nitrogen end. Sometimes, the names make you feel like you’re reading a puzzle’s answer out loud.
The structure itself starts simple: a six-membered ring, like a tiny, chemical Ferris wheel made of carbons and one nitrogen. This backbone, called piperidine, usually pops up in drugs, pesticides, and stabilizers. With this compound, someone’s really packed the ring—methyl groups fill up both carbons right next to the nitrogen, and duplicate that trick on the two carbons furthest away. Layer on top of that two methyl groups glued to the nitrogen, and a single extra amine poking out, and you wind up with a molecule that looks like it should weigh a ton but spins nimbly through chemical reactions.
My experience in chemistry labs always showed me that crowded molecules act like bouncers at a club, blocking unwanted side-reactions. N,N,2,2,6,6-Hexamethyl-4-Piperidinamine isn’t just a random chemical oddity—folk build these types of structures for a reason. Take light stabilizers: the piperidine family keeps polymers from going brittle under sunlight, and hexamethyl substitutions crank up this protection. The density of methyl groups fends off oxygen and free radicals, adding years to plastics or coatings.
It’s a small revelation for engineers and manufacturers. Instead of plastics yellowing in six months, products hold up in playgrounds, car dashboards, and even outdoor signs for years. Behind each sturdy sun visor or long-lasting garden chair, there’s a hidden story about molecular sheltering. This isn’t trivia; sunlight ruins millions of tons of plastic each year, with loss and waste piling up. By understanding and tweaking molecules like this one, we save energy, raw materials, and cut back on pollution from early failure and replacement of plastic goods.
Just because something stabilizes plastics doesn’t mean it’s perfect. Any heavily substituted amine often raises eyebrows for chemists worried about toxicity. I've seen how certain stabilizers, after doing their job, break down and leach into soils and rivers. Sometimes regulatory agencies run years behind the labs in catching up on these risks. The trick sits in balancing durability with environmental safety.
A responsible road forward rests on green chemistry principles. Producers ought to keep watch, tracing breakdown products early and often. Chemists can lean into research for biodegradable analogs, or design structures whose spent fragments don’t linger for decades. Open databases and transparent reporting can nudge everyone—researchers, industry, and policymakers—toward cleaner production without losing out on the durability customers have come to expect.
Molecules like N,N,2,2,6,6-Hexamethyl-4-Piperidinamine offer comfort in everyday living, stretching product life far beyond what we’d get from pure luck alone. There’s satisfaction in knowing which chemical tweaks push society forward, but the greater challenge looms: acting early to dodge environmental harm and push for smarter, safer design. Every little improvement in chemical structure, no matter how jam-packed with methyls, carries that responsibility.
N,N,2,2,6,6-Hexamethyl-4-piperidinamine looks like a tongue-twister, and the name itself hides a complex reality. This chemical comes up often in industrial labs and chemical manufacturing. Companies use it mostly as a stabilizer, especially in plastics and coatings, because it helps prevent damage from sunlight and air. Over the years, it has cropped up in research for textile treatments, paints, even specialty inks. So far, that sounds pretty ordinary. But the substances that keep your garden chairs from turning brittle or your raincoat from fading often deserve a closer look.
Let’s ditch the jargon and dig in: Just handling this substance usually causes trouble if done carelessly. Workers who breathe dust from this compound or get it on their skin can run into irritation. Laboratory safety sheets call for gloves, goggles, and good ventilation, and not just to look professional. People have reported stinging eyes, throat discomfort, and rashes after contact. Like many piperidine-based compounds, this one packs enough punch that a single splash can prompt a trip to the eyewash station. I once spent a day shadowing a plastics chemist; half of our time involved donning gear and checking that no vapor leaks escaped the laminar flow hood. Safety routines aren’t for show: repeated exposure, even at low levels, builds up, causing allergic reactions and blazing up respiratory symptoms.
Lab data and chemical safety agencies like the European Chemicals Agency spell out the risks. Acute toxicity in animals suggests harmful effects by mouth and through skin contact. No, it’s not instantly fatal, but a small amount swallowed or splashed onto open skin can cause lasting harm. Animal tests point to organ toxicity at higher, repeated doses. The U.S. Environmental Protection Agency has flagged its potential for bioaccumulation. That matters, since suspect chemicals like this one do not always leave a person’s body quickly or break down harmlessly in the soil.
Chemicals in the piperidine family sometimes raise concern about chronic health conditions. N,N,2,2,6,6-Hexamethyl-4-piperidinamine lacks decades of population-wide human studies, but the caution flags are not hard to spot. Workers in factories using it stand at higher risk for problems with their livers or skin. The safety data sheets classify it as hazardous, with warnings about reproductive toxicity. Cancer risks stay unclear for most people, but caution pushes workspaces to restrict unnecessary exposure.
Several steps go a long way to keeping workplaces and neighborhoods safe. Strong ventilation systems, personal protective equipment, and real training do more than tick boxes; they make sure that spills or accidents don’t spiral. Regular medical checks for employees uncover early warning signs. National and international guidelines (like REACH in Europe) spell out how much of the substance can enter air or wastewater streams. These limits help protect communities living near large manufacturing sites. Transparency goes a long way too: people living near plants that use this chemical deserve to know if their water or air shows traces, allowing them to demand accountability.
The march toward safer chemistry keeps rolling. Many companies look for UV stabilizers and additives with friendlier safety profiles. Researchers keep hunting for molecules that hold up paints and plastics just as well without the collateral health risks. The story here is familiar—tradeoffs between performance and health run through the history of modern chemistry. But this chemical’s record backs up one simple truth: harder scrutiny stirs up safer practices, and a bit of vigilance lets us protect workers, neighborhoods, and the world beyond.
N,N,2,2,6,6-Hexamethyl-4-Piperidinamine doesn't show up in everyday kitchens or garages, but the people working in labs or manufacturing sites know it brings both opportunity and risk. This compound, sometimes called by its acronym HMPI, pops up in the making of polymers and coatings. Its stability can be an asset, but misuse quickly flips that script. Over years in chemistry labs, I learned that respecting the quirks of each substance builds a culture that prevents injuries and damage, especially with organics like HMPI.
I’ve seen folks underestimate molecules that don't look scary or come with intense warning labels. HMPI lands in that deceptive middle: easy to handle physically, but its fumes and skin effects pack a punch. Its main threats come from skin contact, breathing in vapors, or letting it sit out uncapped. According to the European Safety Data Sheets, HMPI can cause severe irritation and long-term respiratory or eye issues if you throw caution to the wind.
In my own research group, someone once left HMPI near the edge of a bench, without checking the bottle seal. Result? A slick mess and a headache for the crew. Keep it somewhere dry, cool, and out of sunlight. Locking cabinets, preferably with exhaust or airflow, cut down on vapor risks. Don’t lean on assumptions about room temperature—labs can get hot or cold swings, especially if HVAC systems fail. If I had a dollar for every time a storage mishap led to contaminated air or ruined samples, I’d fund a new fridge for my own chemicals.
Shelf segregation matters. HMPI doesn’t get along with acids or oxidizers, so tuck it away from both, not just on a lower shelf in the same closet. Liquid-tight secondary containment pays for itself the first time a bottle leaks. Good labels, date checks, and bottle integrity checks keep everyone in the loop about what’s going on inside that cabinet. A checklist near storage areas helps even the busiest teams avoid routine slip-ups.
Wearing the right gear should be as automatic as tying shoes. Gloves—nitrile over latex for this molecule—protect skin, and splash-proof goggles save eyes. A well-fitted lab coat, not some open jacket, prevents streaks from sneaky splashes. If you need to pour or transfer more than a tiny amount, work in a fume hood. Even knowing all this, I once watched someone try to “just do it quickly” on an open bench and had to remind them: the chemical won’t go easy on impatience.
Spills come with the territory. An emergency kit nearby with absorbent pads, proper neutralizers, and a way to contain the mess stops things from spiraling. Never toss wipes or used gloves in regular trash—they belong in hazardous waste bins. If you get some on skin, immediate washing under tap water makes a huge difference. Safety showers and eyewash stations deserve regular date checks and no clutter blocking access.
Experience shapes safety. Written SOPs help, but nobody learns faster than from a story about what went wrong. Regular reviews keep experienced hands sharp and help new folks see why these precautions aren’t just hoops to jump through. Smart handling of HMPI isn’t about paranoia. It’s about respect—protecting the team, the facility, and everyone’s peace of mind. Chemical work gets safer every time we put in the effort up front, not after the fact.
In chemistry, gaps in purity translate directly into gaps in reliability. N,N,2,2,6,6-Hexamethyl-4-Piperidinamine shows up everywhere from polymer formulation recipes to stabilizer blends. Chemists eye the purity like a chef eyes fresh produce—there’s less room for mistakes with ingredients you trust. Labs stick with grades at or above 98% for work that demands accuracy. In practice, this level satisfies both research trials and quality assurance for end-products relying on the amine’s reactivity.
Purity cuts down on headaches. Lower-purity lots can introduce side-reactions, spike up byproduct contamination, and leave process engineers guessing. Elevated purity over 99% offers technical teams a value worth the premium—cleaner reactions mean less troubleshooting on production lines and less time wasted on purification steps. Synthetic chemists can replace volatile stabilizers in coatings with this amine and expect hard data to match real-world performance, provided purity stays tight.
Anyone handling chemicals knows good packaging matters as much as content. N,N,2,2,6,6-Hexamethyl-4-Piperidinamine doesn’t belong in glorified sandwich bags. Bad seals, porous containers, and ill-suited drums spell bigger issues—think spills, evaporation, or exposure to air that leads to degradation.
Glass bottles hold up in the lab, especially for samples up to 1 liter. Glass won’t interact with the amine and supports more accurate sampling. On the industrial side, steel drums with robust inner linings or high-density polyethylene canisters make sense. They shield from light, resist breaking in transport, and close tightly with leak-proof lids.
Bulk buyers favor drums ranging from 25 to 200 kilograms. A 25-kilogram drum gives plenty for scale-up without turning the warehouse into a hazard zone. Smaller lots—500 ml to 5 liters—let researchers and pilot plant operators cut waste and hold tight quality specs. Producers seeking safety certifications load up their drums with extra features like tamper-evident seals, child-resistant caps, and clear hazard labeling in line with global standards, such as GHS.
Traceability forms the backbone of any responsible chemical supply. Each drum or bottle should sport batch numbers and detailed labeling that call out the producer, date of manufacture, and exact specs. This data gives peace of mind, especially after witnessing recalls where root causes hid in batch inconsistencies.
Stability counts for a lot. N,N,2,2,6,6-Hexamethyl-4-Piperidinamine keeps best in airtight containers, away from acidic vapors, moisture, and direct sunlight. Losing track of storage practices means risking clumpy, yellowed, or chemically changed product—an expensive mistake.
As a chemist concerned with environmental impact, confronting packaging waste feels urgent. Companies that switch to recyclable drums, encourage return-and-refill programs, or design modular packing that scales with need—these outfits stand out. Not only does this cut landfill waste, but it sets new industry expectations.
There’s always space to improve supply chain transparency and accountability. More producers sharing Certificate of Analysis up front, using tamper-proof electronics like RFID for tracking, and partnering with reliable carriers help everyone reduce risk.
Good chemical sourcing means paying attention to details. High purity brings confidence to every batch. Smart packaging supports worker safety and cuts waste. Whole teams win when traceability never slips and materials stay unchanged from factory to loading bay. Investing in all these steps paves the way for better science and practical, lasting results.
| Names | |
| Preferred IUPAC name | 4-Amino-**N**,**N**,2,2,6,6-hexamethylpiperidine |
| Other names |
4-Amino-2,2,6,6-tetramethylpiperidine N,N,2,2,6,6-Hexamethyl-4-piperidinamine Tetramethylpiperidylamine |
| Pronunciation | /ˌɛnˌɛnˌtuːˌtuːˌsɪksˌsɪksˌhɛksəˈmɛθəlˌfɔːr.paɪˈpɛrdəˌniːm/ |
| Identifiers | |
| CAS Number | 3312-60-5 |
| 3D model (JSmol) | `CCCC1(CC(N(C)C)CCN1C)C` |
| Beilstein Reference | 559873 |
| ChEBI | CHEBI:38869 |
| ChEMBL | CHEMBL14301 |
| ChemSpider | 20568241 |
| DrugBank | DB16619 |
| ECHA InfoCard | 100.119.070 |
| EC Number | 2162-98-3 |
| Gmelin Reference | 91715 |
| KEGG | C06044 |
| MeSH | D011515 |
| PubChem CID | 13947 |
| RTECS number | TL9350000 |
| UNII | D1P2L12YI7 |
| UN number | UN2810 |
| Properties | |
| Chemical formula | C11H24N2 |
| Molar mass | 186.32 g/mol |
| Appearance | Colorless to light yellow liquid |
| Odor | amine-like |
| Density | 0.89 g/cm3 |
| Solubility in water | Slightly soluble |
| log P | 1.66 |
| Vapor pressure | 0.32 mmHg (25 °C) |
| Acidity (pKa) | 12.0 |
| Basicity (pKb) | 6.9 |
| Magnetic susceptibility (χ) | -0.000011 |
| Refractive index (nD) | 1.469 |
| Viscosity | 58 cP (25 °C) |
| Dipole moment | 2.95 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 273.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -20.3 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4847.7 kJ/mol |
| Pharmacology | |
| ATC code | D06BX13 |
| Hazards | |
| Main hazards | Causes severe skin burns and eye damage. Harmful if swallowed. Toxic to aquatic life with long lasting effects. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS06,GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | H302, H314, H318, H412 |
| Precautionary statements | Precautionary statements: P264, P280, P301+P312, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | > 99 °C |
| Autoignition temperature | 215 °C |
| Lethal dose or concentration | LD₅₀ (oral, rat): 1690 mg/kg |
| LD50 (median dose) | LD50 (oral, rat) = 900 mg/kg |
| NIOSH | RN 67674 |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 1 mg/m³ |
| Related compounds | |
| Related compounds |
2,2,6,6-Tetramethylpiperidine 2,2,6,6-Tetramethylpiperidin-4-ol 4-Amino-2,2,6,6-tetramethylpiperidine Tempo (2,2,6,6-Tetramethylpiperidin-1-yl)oxyl N,N-Dimethyl-2,2,6,6-tetramethylpiperidin-4-amine |