N-Methylpyrrole sits in a category of chemicals that rose to importance just after World War II, right around the era when chemists started seeing heterocyclic compounds as much more than textbook curiosities. Researchers in organic chemistry circles, especially those working in European labs during the late 1940s, saw promise in nitrogen-based rings. Instead of focusing on established giants like pyridine, they got curious about pyrrole chemistry. By the early 1950s, methylated derivatives were showing up in scientific literature, proving that subtle changes—adding a methyl group, for example—could transform reactivity and application. N-Methylpyrrole started as a bench curiosity, but by the 1970s it had a regular place in organic syntheses in both academic and industrial settings.
N-Methylpyrrole crops up as a colorless or very pale yellow liquid, carrying a faint, sweet-like odor that reminds some folks of nuts. Suppliers usually hand it over in glass-stoppered bottles, with purity anywhere from technical grade to the high-purity stuff required for pharma research. Chemists who’ve worked with it usually remember the liquid’s slick, greasy feel and its ability to blend without much fuss in most organic solvents. There is a regular demand from chemical manufacturers, fine chemistry labs, and the fragrance industry—making it a regular stocking item in many chemical storerooms.
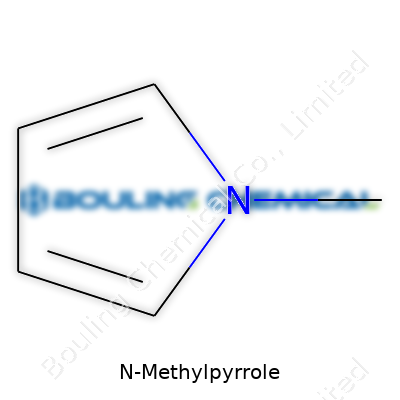
This is a five-membered ring where nitrogen hugs next to four carbons, and a methyl group hangs off the nitrogen. Its formula is C5H7N and it clocks in with a molecular weight of 81.12 g/mol. The flash point sits alarmingly low (around 32°C), which sends a clear message about handling with care. Boiling begins near 110–112°C, and the liquid remains fluid down to about -70°C. Water won’t mix with it much, but put it near ethanol or diethyl ether and it dissolves instantly. If you spill this stuff, the smell sticks around, and its residue sticks to glassware unless cleaned quickly with a strong base and lots of air flow.
Most bottles bear the marks “N-Methylpyrrole” or its CAS number (96-54-8) right on the side. Labels include flash point, hazards about flammability, and a warning about keeping it away from open flames. You’ll see purity listed, with analytical applications demanding 99% or higher. Some containers add storage instructions—cool, dry, tightly sealed. Shipping papers and containers always flag the chemical under Class 3 Flammable Liquids. If you’ve worked with chemical procurement before, you know shipping rules get strict and regulators expect clear labeling and MSDS sheets on hand at all times.
N-Methylpyrrole doesn’t fall out of the sky. Making it in a lab setting usually means starting with pyrrole, then treating it with methyl iodide or dimethyl sulfate in the presence of a base like sodium hydride. The reaction kicks off as the methyl group locks onto the nitrogen, typically at room temperature or a touch warmer. After that initial methylation, extraction brings the product out of the mix, and distillation tightens up the purity. The process throws off significant heat, so cooling baths and well-vented fume hoods take priority; chemists who have tried this once rarely forget how quickly the vapors hit the air.
Compared to its parent compound, N-Methylpyrrole doesn’t offer up its lone pair for hydrogen-bonding quite as easily. This makes it tricky for acid-catalyzed reactions but gives a leg up for certain alkylations and ring-forming reactions. Nitration, bromination, or acylation can all work if you tweak conditions, though the methyl group blocks some routes. Synthetic chemists chase after this compound when developing specialty dyes, as its electron-rich ring gently nudges reactivity in ways unsubstituted pyrrole can’t match. The methyl group bulks up resistance to oxidation, letting the molecule serve as a sturdy building block for more complicated structures.
Over the years, N-Methylpyrrole picked up a few alternate names. You might see it listed as 1-Methylpyrrole, N-Methyl-1H-pyrrole, or even methylpyrrole (1-position specified). In U.S. supply catalogs, the name nearly always appears with the “N-” prefix, cutting confusion with other methylated pyrroles. European traders occasionally use “NMP,” though this can spark mix-ups with N-Methylpyrrolidone, another popular solvent in manufacturing. I’ve noticed catalog numbers change, but the CAS registry keeps the picture clear for ordering.
Handling N-Methylpyrrole calls for vigilance. Safety datasheets flag the liquid as highly flammable, with vapors that can flash in air. Inhalation triggers headaches or nausea, and skin contact may bring burns or irritation. Most labs require gloves, goggles, and—any time I worked with it—a lab coat and splash-resistant apron. Use behind a fume hood, and never open a container near flames or hot surfaces: it evaporates quickly, and one spark can mean a flash fire. Fire safety stations nearby aren’t optional in responsible spaces, and spill cleanup comes with sand or absorbent pads before scrubbing with detergent. Waste usually gets stored in flame-resistant metal drums, headed for incineration.
Industries look to N-Methylpyrrole as a foundational block for specialty polymers, pharmaceuticals, dyes, and flavors. In pharma, chemists leverage its methyl group to fine-tune biological activity—the small change converts mild pyrrole activity into something more robust. Organic electronic materials rely on the ring system as a precursor for polypyrrole-based conductive polymers. Some flavors and fragrances producers use the molecule’s nutty aroma as a starting point for crafting more complex scent or taste molecules. Many dye companies focus on its reactivity for building pigment backbones, exploiting the stability and substitution patterns unique to this methylated pyrrole.
Lab groups always find new tricks with N-Methylpyrrole. Current projects look at greener synthesis routes, aiming to push out hazardous methylating agents for milder, catalytic methods. Polymer scientists keep seeking more controllable polymerizations to create smoother polypyrrole films for sensors or display screens. Some medicinal researchers test methylated pyrroles against antimicrobial strains in an attempt to discover new medicines. I have seen patents for optoelectronic devices and organic solar cells relying on N-Methylpyrrole’s backbone, using the compound as a stepping stone to much bigger molecular assemblies. Most of these projects emphasize how a single methyl group on a nitrogen ring opens doors for new molecular architectures.
Published data point to moderate acute toxicity in animal models, but real-world handling usually brings stronger warnings than the raw numbers might suggest. The methyl group adds a twist; metabolism produces reactive intermediates that can target cellular membranes or proteins if exposure lingers. Chronic studies haven’t found strong carcinogenic tendencies, though long-term inhalation or repeated skin contact stacks up risk for internal organ effects. Essentials here: engineers should design closed systems, regular air monitoring stays mandatory, and personal protective equipment saves trouble in poorly vented areas. No one wants a surprise in the form of nervous system or liver issues traceable to workplace exposure.
Future demand will hinge on two main areas: advanced materials and pharmaceuticals. As wearable electronics evolve, molecules like N-Methylpyrrole could support flexible, electronics-friendly polymers that make sensors smaller and more robust. Drug designers have barely scratched the surface—subtle tweaks of this methylated molecule could yield treatments for neurological or infectious diseases. Meanwhile, demand rises for safer, more efficient synthetic routes that trim out toxic byproducts. From a personal angle, anytime I see a wave of research patents mentioning a core heterocycle, I keep one eye on compounds like N-Methylpyrrole since breakthroughs are as likely to come from a garage lab as a Fortune 500 research bunker.
Ask most people about N-Methylpyrrole and you’ll probably get blank stares. In everyday life, the name doesn’t roll off the tongue, but this molecule quietly shapes many things around us. N-Methylpyrrole belongs to the pyrrole family, which includes compounds found in things as familiar as hemoglobin in blood or in plant chlorophyll. Tweaking the structure a bit by sticking a methyl group on the nitrogen atom creates N-Methylpyrrole, a colorless liquid that scientists consider quite useful.
Drug development rarely gives up its secrets to non-chemists, but N-Methylpyrrole often shows up behind the scenes. Chemists prize its ring structure as a place to build from—it lets them assemble bigger, more complex drug molecules without unwelcome side reactions. That five-membered ring handles transformations well, tolerates a lot of the chemical “roughhousing” chemists put it through, and doesn’t fall apart too easily. This quality keeps it in play during the early steps of making new antibiotics, anti-inflammatories, and even future cancer drugs.
The stuff in our electronics—the flexible displays, high-tech coatings, specialty plastics—all start on the lab bench, and N-Methylpyrrole finds a home here as a raw ingredient. It helps create conductive polymers, especially polypyrrole variations, that power everything from wearable sensors to new kinds of batteries. My own first encounter with these materials came during a college project trying to build a flexible light-emitting patch for bike safety. At the heart of that patch: a polymer chain started with pyrroles very similar to N-Methylpyrrole.
Look at crop protection and plant science, and the story continues. Many modern pesticides and growth regulators trace their ancestry to pyrrole chemistry. Instead of just poisoning pests, some new compounds are fine-tuned to disrupt only targeted biological pathways—thanks to the structural flexibility N-Methylpyrrole provides. Even textile and dye manufacturers use it, exploiting its chemical behavior to produce colors that last longer and resist fading, which matters for clothing and art restoration.
The chemical has its dark side if mishandled. It evaporates fast and releases fumes in the lab, so careful handling and solid ventilation matter to keep exposure risks low. Spills won’t just disappear—cleanup must be prompt and thorough as the compound can irritate eyes and skin. On a broader scale, the growth in new specialty chemicals raises fair questions on how factories handle waste and emissions. More robust controls and better worker training deserve attention, especially since some small compounds slip through filters intended for larger molecules.
Researchers keep looking for ways to make these chemicals from plant-based waste or recycled materials. After all, fossil-fuel derived chemicals drive much of this industry, and supply shortages or high prices can stop research and production dead. Companies that embrace greener processes—say, using bioreactors or creative catalysts—won’t just dodge regulatory headaches. They’ll win favor among buyers who care about the footprint attached to their electronics, medicine, or clothing.
N-Methylpyrrole rarely earns headlines, but anyone involved in chemistry, materials science, or pharmaceuticals owes it a nod. Steps toward safer, more sustainable use don’t rest only on government warnings or safety labels. They grow from chemists’ willingness to rethink old habits, from company budgets for cleaner manufacturing, and from people asking where the ingredients in their everyday products come from. As someone who’s watched innovation up close, I know that change opens doors, and that even a small molecule can lead to big impact.
N-Methylpyrrole doesn’t get the attention of some other chemicals, but in labs and certain industries, it pops up with surprising frequency. Unlike its parent pyrrole, this nitrogen ring carries a methyl group stuck to the nitrogen atom. That one swap delivers a noticeable shift in its chemical personality. If you’re familiar with basic solvents or simple aromatic compounds, you’ll find N-Methylpyrrole carves out its own space with a sweet, somewhat amine-like odor and a tendency to keep to itself, staying liquid at room temperature.
Pouring a small sample of N-Methylpyrrole into a beaker, you’d likely notice the almost colorless or light yellow tinge. The liquid doesn’t thicken up like syrup; it runs more like water. At just over 100°C, it boils away—much lower than what you’d see with the non-methylated pyrrole. Chemists in the lab don’t need to crank the heat too high to distill it, so that’s a plus for handling and storage. It mixes well with classic organic solvents, so there’s no major headache in separation or purification.
With a density that lands near 0.98 g/cm3, it behaves a lot like many organic solvents you’d find on the bench. It won’t freeze on a chilly winter day since its melting point—somewhere under -60°C—keeps it liquid well below freezing. In practical use, that translates to fewer worries about accidental crystallization or phase changes if your workspace cools off.
Adding that methyl group gives N-Methylpyrrole a boost in stability compared to pyrrole itself. Air and light won’t break it down right away, but over time or under tough conditions, it can still oxidize. If you’ve worked with pyrrole, you’ll spot the difference in how fast the methylated version forms those pesky tars or colored byproducts. It holds up better and doesn’t polymerize as fast if you leave it in the open air.
Chemistry students might recall that pyrrole’s nitrogen atom loves to share electrons, feeding reactions from electrophilic substitution to ring fusions. N-Methylpyrrole tampers down that urge a bit. Since the nitrogen’s lone pair no longer sits fully exposed, thanks to the methyl group, the molecule resists some of the classic electrophilic attacks. Nitration and bromination take more coaxing. Anyone seeking to run Friedel-Crafts reactions will hit fewer surprises, but sulfonation or chlorination becomes less straightforward. Still, the nitrogen keeps its basic character, making the compound a handy intermediate for those building more complex molecules.
N-Methylpyrrole stands out in manufacturing certain pharmaceuticals and advanced polymers. In the right hands, chemists exploit its electronic quirks to build specialty organic materials. Its relatively low boiling point and stability allow efficient purification, a big relief compared to juggling more delicate, reactive substances. Still, its volatility means any spill quickly fills the air with a strong, unpleasant odor, and close contact invites skin and eye irritation. Everyone in the lab learns to handle it under a fume hood soon enough.
Disposal and storage ask for care, too. Metal containers risk corrosion over time if tiny leaks allow air in and oxidation gets started. Glass, tightly sealed, works better for longer shelf life. For environmental safety, used N-Methylpyrrole and contaminated equipment should run through approved disposal systems. Regulations often group it with other mildly hazardous solvents, so shortcuts don’t pass inspection.
Lab training pays off. Everyone who handles N-Methylpyrrole should run drills on spill control and venting. Shifting away from bulk storage to smaller, sealed ampoules cuts down risk. Moisture and exposure controls also help—simple silica gel packs in storage cases go a long way. For manufacturers, tighter exhaust filtration and real-time monitoring limit environmental impact. As more research unlocks milder synthetic methods and cleaner derivatives, safer alternatives may eventually outpace N-Methylpyrrole, but for now, knowing its chemistry and taking everyday precautions keep its risks in check and its benefits flowing.
N-Methylpyrrole sounds like one of those chemicals you’d mostly encounter in a lab coat, but it sneaks into real-world applications as a solvent or building block for other compounds. Anyone who spends time around solvents knows how regular folks can take safety for granted. People might open a drum without thinking twice. It's easy to ignore precaution when a chemical doesn’t smell too strong or make your skin sting right away.
N-Methylpyrrole isn’t considered the most infamous chemical on the block, but that doesn’t mean you can treat it like saltwater. Spend some time reading through material safety data sheets and you'll see this chemical can irritate skin and eyes. You get this stuff on your hands a few times and the dryness sneaks up, sometimes a rash. Breathing in vapors left me lightheaded before, so ignoring those fumes feels like rolling the dice.
Testing in animals shows mild toxicity by ingestion. This doesn’t mean people can ingest a tiny splash and walk away just fine—chemicals behave differently in each body. Plus, if you have open wounds or tend to touch your face, accidental exposure can quickly spiral beyond “just a little.” My coworkers used to say, “It won’t kill you fast, but why take the risk?” That echoes in any reasonable workplace.
Years back, I watched someone work with N-Methylpyrrole in a cramped storage room with no fan, wearing single-use gloves and regular glasses. The next day, they had itchy eyes and a headache that wouldn’t quit. If you're pouring out a liter or two, gloves and goggles aren’t overkill—they feel more like common sense.
Ventilation—the mundane hero—does most of the heavy lifting. I’ve felt the difference between closed and open windows working with these kinds of chemicals. Even a table fan pushing fumes away works in a pinch, though a proper fume hood beats any hack. Don't wait for symptoms to show up. The habits you form after the first few exposures stick with you and keep accidents from happening.
Storing N-Methylpyrrole tends to reveal who plans ahead and who wings it. Leaving the container open—or letting vapor build up—feels tempting because the liquid itself doesn’t reek. It still evaporates. Containers should get tightened, labeled, and stowed in a cool spot, away from the lunch fridge. I’ve ended up cleaning up one too many chemical leaks because someone left a lid loose. Absorbent pads and lots of fresh air fix most small spills, but big ones demand the spill kit and time off the clock.
Some think a chemical with a modest hazard rating barely matters. That way of thinking leads to careless mistakes. I remember a seasoned tech telling me to treat every substance like it’s worse than advertised. It isn’t excessive. Simple steps like regular glove changes, washing up after you finish the job, and having clear protocols for accidents don’t slow anyone down in the long run. The safest lab or workshop doesn’t just nod to regulations—they make basic caution part of the routine.
People often ask if one chemical is dangerous. That misses the real point: it only takes a slip to realize how fragile safety can be. Handling N-Methylpyrrole with respect—ventilation, gloves, eye protection, and smart storage—lets you get the job done and walk away healthy. Chemistry isn’t just what happens in a flask, but what you remember after you wash your hands and call it a day.
The world of chemistry doesn’t always hand out simple puzzles. One that grabbed my attention early on was N-Methylpyrrole. For the uninitiated, its molecular formula is C5H7N. Not the most complex combination, but don’t let that fool you. This molecule comes packed with interesting quirks. The moment you scribble it on paper, you see five carbons strung together in a ring, four hydrogens welded to the carbons, another hydrogen locked onto the ring nitrogen, and a methyl group clinging tightly to that same nitrogen atom. Everything fits just so.
Diving into structure is where N-Methylpyrrole separates itself from standard ring compounds. Think of it as pyrrole with an extra methyl group. Take pyrrole, a five-membered ring with one nitrogen. Now, add a methyl group at the nitrogen site. No magic tricks—just basic chemistry. This little tweak flips some major switches in reactivity and properties.
N-Methylpyrrole shows up in a lot of synthetic chemistry. It’s got that classic aromatic five-membered ring, so it’s flat, stable, and resists breaking apart unless you push it hard. Attaching the methyl group where the nitrogen sits gives it a character all its own. The electron cloud spread across the ring changes. That methyl group bulks up one side, shifting electron density. Suddenly, places that used to attract interest from other molecules react differently. Synthesis guys take note: swapping out plain pyrrole for the methyl version means you sometimes get a whole new set of possibilities.
Folks in labs lean on this molecule when they’re piecing together more complex targets, especially pharmaceuticals and advanced polymers. Its altered structure can give bioactive molecules longer shelf lives or change how they interact inside the body. In the real world, these tweaks can decide if a new medicine cures you or sits useless on a shelf.
As a chemistry student, I once had to run a reaction designed for pyrrole, not the methyl version. Let’s just say, the workup was full of surprises. The methyl group changed the boiling point, shifted the yields, and the compound’s solubility moved just enough to mess with purification. Playing with structure isn’t an academic exercise. It means someone in a factory, or down the hall in a hospital, gets better results from what we make.
The fun doesn’t stop at just spotting the additional methyl group. There’s a deeper point: even tiny molecular changes shift everything from toxicity to environmental fate. Regulators don’t treat N-Methylpyrrole like pyrrole. Waste disposal? Safety data sheets? It all shifts over a single -CH3 stuck to nitrogen.
Cautious chemists eye these differences. Some watch for better drug candidates, others for novel materials. Pushing research means staying awake to these details. The way a molecule’s shape matches with another—whether in a flask or a receptor in the body—can rewrite the story for researchers, doctors, and patients.
As a solution, schools should teach students real-world lessons about chemical structures. Instead of just rote memorization, focus on why each atom sits where it does, and how a methyl group transforms function. Mixing practical know-how with molecular sketches bridges the gap between blackboard and breakthrough.
N-Methylpyrrole doesn’t fill headlines, but anyone working around chemicals learns pretty quick that each substance brings its own list of headaches. This one gives off flammable vapors. Even as someone who’s been around the block with solvents and reagents, I’ve seen storage rooms get aromatic from carelessness. N-Methylpyrrole—like plenty of organic chemicals—demands respect. Mishandling can risk fire, employee exposure, and even busted containers.
Years in labs taught me that it’s never enough to simply toss bottles on any empty shelf. Cabinets built for flammables usually have steel construction, grounded doors, and strong ventilation. That’s what N-Methylpyrrole claims as its sweet spot. I’ve watched fires pop up from sparks and static discharge, so having those grounded storage units means less drama. It’s worth checking that the cabinets stand away from direct heat sources, whether it’s sunlight sneaking through a window or a rogue space heater nearby.
Temperature swings can make some chemicals sweat, build up pressure, or even burst their seals. N-Methylpyrrole prefers cool, stable places—away from the kind of cold that cracks bottles, but nowhere near anything resembling a hot roof in summer. Humidity might seem harmless, but moisture can grind through caps or trigger unwanted side reactions. Keeping it dry is more than a suggestion, it’s part of keeping things quiet and predictable.
Folks often think twice about what’s clanking in a delivery van, but overlook the chaos caused by bad bottle seals or leaky containers. Double-checking caps every single time beats mopping up a spill at the end of the day. Labels sometimes peel or fade, but sharp, obvious warnings matter. Respiratory gear and gloves should become muscle memory, not an afterthought. I’ve seen new hires skip the gloves—until their skin starts stinging. That’s all it takes to make safety rules stick.
Flooding a room with fumes is the fastest route to evacuation. Even a slow leak will hang heavy without proper airflow. Investing in exhaust systems and regular checks for every stockroom turns what might feel like an unnecessary expense into cheap insurance in the long run.
Rigged-up moving trucks and slapdash crates have no place in shipping flammable liquids. I’ve watched folks trust cheap cardboard, only to find containers rolling out of broken boxes. Wooden crates or steel drums with chemical-resistant linings offer real protection. Add shock-absorbent padding, and everything inside sits tight even if roads get bumpy. Certified containers cost more, but I’ve never regretted springing for the good stuff.
There’s always paperwork to handle, which nobody enjoys, but every form and sticker makes it less likely anything gets misplaced or mishandled. Regulations can seem dense, especially with the ever-changing rules for hazardous material transport, but ignoring them leads to fines bigger than any compliance bill.
Every ounce of preparation beats an emergency. N-Methylpyrrole isn’t some monster, but treating it casually rarely ends well. Equipment checks, emergency spill kits right by storage doors, and a living written plan for messy surprises turn accidents into minor stories instead of disasters. Workers showing up for their shifts with the mindset that their health and the company’s bottom line both benefit from good storage and solid transport—those are the labs and warehouses worth copying.
| Names | |
| Preferred IUPAC name | 1-Methyl-1H-pyrrole |
| Other names |
1-Methylpyrrole N-Methyl-1H-pyrrole |
| Pronunciation | /ˌɛnˌmɛθəlpaɪˈroʊl/ |
| Identifiers | |
| CAS Number | 120-94-5 |
| Beilstein Reference | 1465062 |
| ChEBI | CHEBI:37975 |
| ChEMBL | CHEMBL32150 |
| ChemSpider | 138690 |
| DrugBank | DB04258 |
| ECHA InfoCard | 100.016.835 |
| EC Number | 01-2119987855-13-0000 |
| Gmelin Reference | 63577 |
| KEGG | C01718 |
| MeSH | D010016 |
| PubChem CID | 7927 |
| RTECS number | UE7175000 |
| UNII | C7M4O79M55 |
| UN number | UN2810 |
| Properties | |
| Chemical formula | C5H7N |
| Molar mass | 81.12 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | amine-like |
| Density | 0.967 g/mL at 25 °C |
| Solubility in water | slightly soluble |
| log P | 0.86 |
| Vapor pressure | 3.7 mmHg (20 °C) |
| Acidity (pKa) | 23.3 |
| Basicity (pKb) | 6.77 |
| Magnetic susceptibility (χ) | -5.76 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.495 |
| Viscosity | 0.774 cP (25 °C) |
| Dipole moment | 1.74 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 143.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -23.9 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3024 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | N05CM23 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Precautionary statements | P210, P280, P304+P340, P312, P403+P233 |
| NFPA 704 (fire diamond) | 2-3-0 |
| Flash point | 34 °C |
| Autoignition temperature | 315 °C |
| Explosive limits | 1.8–10.4% |
| Lethal dose or concentration | LD50 oral rat 695 mg/kg |
| LD50 (median dose) | LD50 (median dose): 1070 mg/kg (rat, oral) |
| NIOSH | No data |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 mg/m3 |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
Pyrrole N-Ethylpyrrole N-Methylindole Pyrrolidine |