N-Methylimidazole didn’t pop up overnight in laboratories. Chemists started paying attention to the imidazole ring back in the late 1800s, hunting for compounds that could speed up reactions or mimic enzymes. I remember pouring through dusty journals in college, reading about early methods that required glassware most chemists now consider artifacts. Early synthesis involved pretty brute-force methods with methylating agents, before green chemistry and safety protocols made things more refined. What started as curiosity about heterocyclic chemistry turned into foundational work that brought N-Methylimidazole to all sorts of labs, from pharmaceuticals to electronics. Its widespread adoption has had less to do with sudden breakthroughs and more about how reliably it has turned up solutions, project after project, decade after decade.
What does N-Methylimidazole promise to chemists today? It’s a colorless or pale-yellow liquid, pretty stable in a bottle, with a subtle amine odor that often serves as a first whiff warning about keeping the cap on tight. Its rise in use owes much to how conveniently it dissolves in water and organic solvents, allowing it to slot into processes without drama. I’ve always thought of it as the chemistry equivalent of masking tape: unglamorous but absolutely essential, holding together transition metal catalysis, being the backbone in pharmaceuticals, and showing up as a building block for molecular tweaks that make bigger reactions work.
This liquid boils at around 198°C and chills to solid only well below room temperature, making it handy under a variety of setups. Unlike some finicky reagents, N-Methylimidazole resists decomposition, playing nice in the company of acids, bases, and most solvents. It’s got a pKa close to 7, not far from water, so it often acts as a gentle base that doesn’t start fires or rust equipment. Its chemical makeup—one more methyl stuck on the imidazole ring—gives it slight hydrophobic character, but never enough to keep it out of water-based chemistry. Handling it outside the hood, even for a minute, will have you regretting its stubborn aroma sticking to your gloves.
Labels spell out its CAS number (616-47-7), chemical formula (C4H6N2), and batch purity—usually sitting at 99% or better for lab use. I've checked packaging plenty of times, most manufacturers stamp on hazard statements like “Harmful by inhalation” and demand gloves and goggles. Specifications outline maximum metal content, color clarity, and water traces down to parts per million for some electronics applications. Despite rigorous detail, errors happen—anyone who’s ever re-checked a bottle knows that even reputable suppliers can’t keep every impurity out.
Making N-Methylimidazole involves methylating imidazole, often using methyl iodide, dimethyl sulfate, or methyl halides, depending on the plant or country regulations. Decades ago, safety teams worried about accidental spills, since methylating agents bring their own hazards. Modern setups use less volatile agents and stricter containment, but homemade runs, like those I saw in a grad school lab, always involved nervous glances. Finished product comes out through vacuum distillation or crystallization, with safety teams fiercely monitoring vent lines. The overall goal—make the methylation happen cleanly without blowing up the building or contaminating the product with junk that ruins downstream reactions.
N-Methylimidazole gets called up in reactions needing a mild base. It's especially good at encouraging acylations and alkylations without wrecking sensitive molecules. Its electron-rich nitrogen lets it coordinate to metals—palladium, copper, rhodium—and boost catalytic cycles in cross-coupling reactions, which anyone making complex pharmaceuticals has come to rely on. I have used it to coax lazy reactions forward or to mimic enzyme cofactors. The methyl group narrows the window for further ring tweaking, but that’s a worthwhile trade-off for the stability and selectivity gained. In some bioconjugation steps, adding just the right batch of N-Methylimidazole makes the difference between a nice, clean product and a bottle full of useless goo.
It has plenty of aliases: 1-Methylimidazole, NMI, and even “N-methyl-imidazol” in some older German catalogs. Different suppliers create their own trade names but the backbone remains: every serious chemical catalog gives you the imidazole ring, methyl stuck on nitrogen, along with purity stats and safety icons you ignore at your own peril.
N-Methylimidazole isn’t gentle to skin or lungs; gloves, goggles, and a fume hood stand as non-negotiables. Bigger production plants add sensors and scrubbers because even brief inhalation causes headaches, and long exposure risks organ effects. I’ve seen a colleague dunk a pipette in an open bottle—his irritation reaction sent ripples through our safety meeting schedules for months. Labels back up these personal experiences, flashing GHS signs and warning about chronic exposure. MSDS sheets list long, sometimes alarming pages of what happens in spills or fires, but with good practices embedded in every step, risks stay manageable. Waste streams route through specialized solvents or incineration, with constant monitoring for leaks.
My lab’s first real experience with N-Methylimidazole came through pharmaceutical synthesis, where it drives peptide couplings and nucleoside transformations that form the backbones of antiviral and cancer drugs. Colleagues in battery labs borrow it for ionic liquid design to boost charge capacity, while those in agrochemical startups rely on it for specialty herbicide intermediates. Its coordination chemistry keeps it close to catalyst development, producing everything from OLED displays to drug precursors. In biotechnology, N-Methylimidazole partners with nucleotides and enzyme mimics, delivering yield improvements in million-dollar processes. Its role stretches across organic routes, always showing up where gentle yet effective base activity is needed.
New uses for N-Methylimidazole crop up with every yearly literature review. Custom derivatives headline journals chasing selective catalysis, greener processes, or reshaping metabolic pathways. Development teams churn out analogs to tweak water solubility or reduce toxicity, answering calls from regulatory agencies and eco-conscious investors alike. My run-ins with custom derivatives often revolve around troubleshooting reaction quirks. Chopping a methyl group to an ethyl or installing a halogen sounds easy but routinely triggers a headache during purification stages. Every workday, a fresh paper pops up promising lower waste or faster synthesis using variants of the same methyl-imidazole backbone. As technical standards evolve, so do industry targets—yields get pushed, energy budgets shrink, greener solvents beckon. N-Methylimidazole’s adaptability keeps it firmly anchored in journals and startups.
Animal studies flag moderate toxicity—doses causing liver and kidney effects demand serious respect during handling. Regulatory agencies require robust long-term exposure studies; I have encountered safety discussions where workers with repeated exposure developed odd health complaints, flagged and eventually tracked to improper fume hoods. Ecotoxicology tests find some danger to aquatic life. Institutions keep setting lower and lower workplace exposure limits as precaution. Most labs track airborne concentrations with personal samplers, logging exposure down to fractions of a part per million. In academic labs, outreach efforts always stress gloves and good ventilation, and there’s a slow shift to replacing N-Methylimidazole in basic student courses just to avoid accidents.
Looking ahead, green chemistry keeps tugging at process chemists to find alternatives or improve recovery. N-Methylimidazole stands a strong chance of sticking around, thanks to its unique mix of solubility and reactivity. Electronics and pharma sectors will keep scaling up use, especially with flow chemistry and new automation. Industry trends point toward recycling spent NMI through advanced purification rather than discarding it as hazardous waste. Researchers aiming to reduce toxicity are developing ring-modified variants, seeking to dial back unwanted effects while keeping catalytic activity high. Any new regulatory hurdles will push production plants toward tighter controls and lower emissions, giving chemical engineers plenty to work on. Most academic research aims to balance synthetic flexibility with environmental goals, hoping to keep N-Methylimidazole’s strengths while cutting its risks. Even with competition from fancy designer molecules, its familiarity and versatility guarantee a continued role in science and industry—just as it has for decades.
N-Methylimidazole isn’t a name most folks kick around at dinner, but it shows up all over modern industry. This little compound forms the backbone for more reactions than you might guess. Its popularity springs from its unique ring structure—a nitrogen-rich five-membered ring that makes chemists smile because of its stability and reactivity.
Factories and research labs reach for N-Methylimidazole because it acts as a solid helper in pushing tough reactions along. My experience working in a fast-paced lab gave me a front-row seat to its use. We relied on it as a base and as a catalyst, especially in pharmaceuticals. Big drug companies love it because it helps them fabricate new medicines without the usual hurdles. The molecule gets involved in the kind of chemistry most medicines need, slipping into those reactions where other chemicals fumble.
Look at any recent drug synthesis journal and you’ll find a shout-out to N-Methylimidazole. It spins the wheels in creating antibiotics, antivirals, and cancer drugs. During an internship at a drug development company, I became familiar with the never-ending pressure to squeeze every drop of value from raw materials. N-Methylimidazole helped us accomplish transformations in a single step that used to take three or four. That means medicines arrive faster, sometimes safer, and—often—a bit cheaper.
N-Methylimidazole has bigger dreams than just pharmaceuticals. The chemical industry relies on it to manufacture dyes, corrosion inhibitors, and, notably, to enhance electrolytes in batteries. Workers in the energy storage field know this molecule as a silent hero. Battery efficiency continues to define how long our phones last or how far an electric car travels. The small improvements made by adding compounds like N-Methylimidazole carry enormous consequences for consumers.
When it comes to manufacturing specialty chemicals, this compound fills an important role. For example, resin production depends on it for improving setting times and durability. Electronics manufacturers look to it for producing essential building blocks for computer chips. The world of agriculture also uses it for making agrochemicals that support higher crop yields.
N-Methylimidazole has its dark side. It brings toxicity risks if folks treat it carelessly. Research shows inhaling or ingesting even small amounts can cause health effects. Factories store and transport it in sealed tanks, and safety teams monitor for leaks or spills. I’ve seen the drill—workers in gloves and face masks, sensors on every wall. Regulations are getting stricter, and for good reason.
Companies and labs already work on greener alternatives, aiming to slash reliance on potentially toxic chemicals. Some researchers are looking into bio-based catalysts that could replace N-Methylimidazole without sacrificing performance. For now, careful handling and proper disposal lower the risks. Responsible supply chains and investment in worker protection keep this essential tool in the chemistry toolbox without putting too many people or ecosystems in harm’s way.
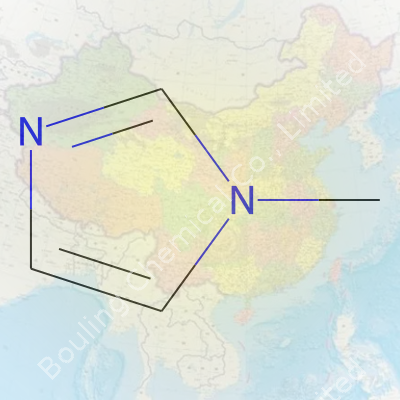
Ask any chemist about N-Methylimidazole, and you’ll probably notice a certain fondness in their voice. Here’s a molecule that gets plenty of use, whether in drug development, lab research, or industry. Its chemical formula is C4H6N2. Nothing outlandish—just the right mix of carbon, hydrogen, and nitrogen given a bit of a twist by the methyl group sticking out from the main imidazole ring. The molecular weight clocks in at 82.10 grams per mole. Precise, but what really matters is what that weight and formula let us do.
Years on a research bench will teach you to appreciate reliable reagents, and N-Methylimidazole earns a spot on many shelves. Its structure isn’t complicated, but don’t let that fool you; it’s got flexibility. The methyl group tweaks its properties just enough to make it both practical and versatile. Pull this compound off the shelf, and suddenly tough jobs—like acylation or alkylation reactions—start looking a little easier. It handles nucleophilic catalysis like a champ, and can help solvents face trickier reactions. In labs where every gram and every reaction counts, that matters.
You’ll find N-Methylimidazole mentioned in stories about pharmaceuticals and green chemistry. A colleague once told me he’d rather run out of coffee than this molecule—which says something, since caffeine often seems like a scientist’s core fuel. Why do so many people value it? One big reason: its consistency. It’s predictable, it’s not volatile or temperamental, and it gives clear results. That lets researchers focus less on troubleshooting mysterious side reactions and more on driving research forward.
Easy as it is to focus on reaction yields or theoretical use, it’s hard to ignore the health and safety side. Spend enough time in labs and you learn to treat all chemicals with respect. N-Methylimidazole does carry some hazards—skin irritation, possible toxicity when inhaled or ingested, and the environmental impact of larger-scale disposal. Labs and manufacturers have a role to play here, making sure protective equipment is no afterthought, and chemical waste isn’t just poured down a drain. Training, proper storage, and careful handling cut risks way down.
Is this the only compound that does the job? Not always. There’s a steady drumbeat for greener and safer chemistry. Labs could replace N-Methylimidazole in certain processes by switching to other heterocyclic compounds or even enzyme-based catalysis where possible. Yet the search for replacements can grow complicated, as new reagents invite fresh troubleshooting and sometimes bigger headaches. Until something better comes along, this compound will keep showing up wherever synthetic and pharmaceutical teams gather.
Chemistry never really stands still; reagents get swapped, methods improve, and what seemed essential ten years ago might drop out of favor. Right now, with its formula and manageable molecular weight, N-Methylimidazole remains a real workhorse. Balancing safe handling and environmental concerns alongside ongoing research into alternatives looks like the best path forward. In science, reliability leaves a mark, and so far that’s the legacy of this simple C4H6N2.
N-Methylimidazole often pops up in labs and workplaces that deal with specialty chemicals. It isn’t a name most people hear outside those circles, but the question about its safety reaches beyond the walls of a lab. I’ve seen chemicals dismissed as “routine” snap back because their risks weren’t respected. Maybe that’s why, after seeing a splash on a lab coat or smelling something sharp in the air, I look closer at anything new on the shelf.
Let’s lay out some facts: N-Methylimidazole comes with a health warning for a reason. Handling it without gloves can leave skin irritated. Breathing it isn’t recommended, either. A few studies say that inhaling the vapors may irritate the nose and lungs. Swallowing or getting a heavy dose on your skin can cause headaches, dizziness, or nausea. Prolonged exposure can do more serious damage: it’s been linked to liver and kidney issues in animal tests. All of this boils down to a commonsense conclusion—anyone working near this material owes themselves the favor of reading the MSDS sheet and following real safety steps, not just filing the document away.
In the rush to finish a synth or keep the plant moving, corners get cut. I’ve watched old hands toss their gloves on a bench, claiming, “I’ve handled worse.” Years in—sometimes decades—can make people too comfortable with risk. The flip side is someone brand new, nervous enough to treat every unlabeled bottle like it’s radioactive. Balance comes from treating N-Methylimidazole as what it is: a useful tool that deserves respect.
Anyone tinkering with chemicals knows accidents don’t always stay inside the fume hood. Leaks and spills can lead to exposure far from where people expect them. I remember a warehouse mishap where a poorly sealed drum corroded through. You get one chemical floating through the air, everyone in the building gets a headache, and suddenly nobody’s dismissing MSDS warnings. Compounds like N-Methylimidazole don’t spread easily unless something’s gone wrong, but when they do, it’s never controlled.
It’s not only about the people handling the compound directly. Downstream risks matter: improper disposal can hit water tables or soil. These things turn up in the oddest places when maintenance slips or records get sloppy, and once it’s in the wrong spot, clean-up costs pile up quickly. Environmental monitoring groups have written that imidazole compounds, including N-Methylimidazole, stick around unless they’re properly contained and treated. Chronic exposure—meaning small amounts over weeks or months—can have health effects even if no one ever gets acutely sick.
There’s no fancy trick to handling a chemical like this safely. Training can’t be a one-off event. Everyone in the chain, from the receiving dock to the chemist, needs to know the real hazards and see the right equipment used for the job. I’ve seen the difference between shops with a living safety culture and places where the eyewash station collects dust. One approach works; the other is just hoping for luck.
Anyone who says N-Methylimidazole “isn’t that bad” ignores what happens when people cut corners. Safe storage, regular inspections, and clear labeling have to matter at every level. The people on the ground—literally handling the stuff—deserve more than a pile of paperwork and vague rules. Real safety flows from direct, honest conversation and practical steps, not just ticking off compliance boxes.
N-Methylimidazole doesn’t show up in daily headlines, but for a lot of labs and chemical facilities, its safe storage can mean the difference between a good day and a risky one. The stuff isn’t one of those household names, yet walk through any real research environment or an industrial lab, and you’ll probably spot a brown bottle of it tucked away on a lower shelf. I spent years in chemical storerooms before the age of digital inventory tracking. Back then, knowing how to store chemicals didn’t depend on a QR code—you learned by watching a more experienced tech or by seeing what happened when something went wrong.
N-Methylimidazole gives off a strong, fishy odor. That alone tells you it’s volatile. Its low flash point alongside its ability to catch fire means keeping it near a source of ignition isn’t worth the gamble. Even a quiet storeroom isn’t immune to mishaps. A tipped bottle or a leaking cap can lead to fumes, headaches, or worse, a fire. I remember a storeroom mix-up where a solvent bottle wound up next to acids by mistake. No one got hurt, but the scare prompted a quick overhaul of our labeling process.
Exposure also means something: spilled N-Methylimidazole soaks into your skin or evaporates into the air quicker than you might expect. Even seasoned pros get careless on busy days, and suddenly that faint odor catches in your throat. You want that bottle tightly closed and nestled somewhere cool, away from sunlight or anything that sparks.
Stick it in a flammables cabinet. Simple fix, but not everyone does it. You’d be shocked at how many times people stack strong solvents on a crowded open shelf just to clear some space. Flammables cabinets, made of steel with a self-latching door, give you a fighting chance if something spills or if there’s a fire.
Keep the bottle sealed. Those vapor caps make a real difference, especially compared to the old push-and-twist closures. If you handle the chemical often, grab safety gloves and maybe eye protection. Label everything clearly: half my old labels faded so badly you could barely read them, and that confusion breeds accidents.
Temperature stands out as a storage factor. Most storerooms run at room temperature, but hot summers push those rooms way above comfort. I’ve seen chemicals become more volatile as the seasons change, so if the building heats up, keep chemicals in the cooler cabinets or areas where air moves freely. Humidity and wet storage make things worse, so dry shelves away from sinks and water lines serve your best interests.
Plenty of issues fade by taking a few easy steps seriously. A dedicated spot for chemicals like N-Methylimidazole, good airflow, and a locked cabinet go a long way toward keeping a workspace safe. Training new folks, even with just a walkthrough of the storage area, helps reduce dumb mistakes.
Handling hazardous chemicals isn't just about keeping up with the regulations—it’s about watching out for your own skin and everyone working beside you. If you’ve smelled N-Methylimidazole once, you won’t forget it, and that quick whiff serves as a reminder for why proper storage never goes out of style.
You only really appreciate the difference purity brings when you’ve wrangled with cheap chemicals that gum up your glassware or knock an experiment off-course. N-Methylimidazole walks that tightrope, and purity makes all the difference. Try running a reaction with technical grade instead of high purity—you’ll see the results, sometimes in a cloud of puzzling byproducts or slow yields that eat up your patience. Folks in the lab know how fast an impure batch turns a full day’s workload into a tangled mess.
Most suppliers break it down into three main grades: technical, laboratory (or analytical), and high purity (sometimes called high-performance liquid chromatography or HPLC grade). Technical grade hovers around 95–98%. This is what you’ll find in larger drums, usually destined for the paint, polymer, or industrial solvent crowd. It’s affordable. That comes with a fair share of water, leftover reactants, or rogue byproducts. On the production line, those traces may not bother anyone, especially if the solvent’s washing off later anyway.
Lab grade usually runs about 98–99%, and you’ll spot it in most university storerooms and in R&D departments where budgets squeeze every penny but still leave some room for decency. At this range, you sidestep major interruptions from impurities, so structure identification and routine synthesis go more smoothly. That extra one or two percent can save a week by sparing the need to re-run a botched reaction.
High purity grades (99.5% or above) top the list for sensitive work. Think pharmaceutical synthesis, catalyst research, high-stakes electronics, and anything that punishes contamination. I’ve watched colleagues pay twice as much for a reagent in this category, just to avoid unpredictable peaks when running NMR spectra or chromatography. The point isn’t snobbery—trace contamination knocks precious samples off course. An extra half-percent of purity can mean a night’s sleep instead of troubleshooting til dawn.
You can test the limits by trying to substitute a cheaper grade in a critical step, just don’t expect flattering results. In practice, those low-level impurities will corrode catalysts, foul up columns, and cost time. Once I had a bottle labeled 98%—on paper, that sounds pretty clean. In practice, the mystery two percent made my product oily and impossible to purify. Eventually, I traced the problem to the cheap starting material. I learned fast—cutting corners here means losing elsewhere.
For me, the most helpful thing would be clearer labels and more detail in certificates of analysis. Lots of companies throw out broad claims—“suitable for synthesis,” “research use only.” What does that mean? Which impurities? In what amounts? Reliable suppliers go out of their way to spell it out. Too many others stay vague, especially at lower price points. I wouldn’t mind tighter oversight either, so a 98% actually means 98%, not “roughly.” Scientists want to solve problems, not spin their wheels doubting what’s in the bottle.
N-Methylimidazole’s three main grades—technical, laboratory, and high purity—fit real-world needs across chemistry, manufacturing, and research. Every level plays a role, but trying to stretch one tier for the demands of another usually ends in frustration. The right grade pays for itself, in good yield, reproducibility, cleaner samples, and less wasted time. Industry could do with better transparency and less hand-waving—because nobody wants to gamble their next experiment on what’s left unsaid.

| Names | |
| Preferred IUPAC name | 1-Methyl-1H-imidazole |
| Other names |
1-Methylimidazole NMI Methyimidazole 1-Methyl-1H-imidazole |
| Pronunciation | /ɛnˌmɛθ.ɪl.ɪˈmɪd.əˌzoʊl/ |
| Identifiers | |
| CAS Number | 616-47-7 |
| Beilstein Reference | 85390 |
| ChEBI | CHEBI:18434 |
| ChEMBL | CHEMBL1539 |
| ChemSpider | 6437 |
| DrugBank | DB02145 |
| ECHA InfoCard | 100.023.460 |
| EC Number | 214-672-9 |
| Gmelin Reference | 7247 |
| KEGG | C06575 |
| MeSH | D008008 |
| PubChem CID | 6978 |
| RTECS number | NT0700000 |
| UNII | J3CZ4751I2 |
| UN number | 2925 |
| CompTox Dashboard (EPA) | `DTXSID3020405` |
| Properties | |
| Chemical formula | C4H6N2 |
| Molar mass | 82.11 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | amine-like |
| Density | 1.03 g/mL |
| Solubility in water | miscible |
| log P | 0.02 |
| Vapor pressure | 0.53 mmHg (20°C) |
| Acidity (pKa) | 7.05 |
| Basicity (pKb) | 7.15 |
| Magnetic susceptibility (χ) | -32.8·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.488 |
| Viscosity | 1.232 cP (25 °C) |
| Dipole moment | 3.67 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 181.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | −34.9 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -372.6 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | N03AX17 |
| Hazards | |
| GHS labelling | GHS02,GHS05,GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Danger |
| Hazard statements | H302, H314, H317, H373 |
| Precautionary statements | P280, P261, P305+P351+P338, P304+P340, P312, P337+P313, P501 |
| NFPA 704 (fire diamond) | 2-3-0 |
| Flash point | 58 °C |
| Autoignition temperature | 464°C |
| Explosive limits | Explosive limits: 2.2–12% |
| Lethal dose or concentration | LD50 oral rat 970 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 820 mg/kg |
| NIOSH | NIOSH: PA0830000 |
| REL (Recommended) | 100 ppb |
| IDLH (Immediate danger) | 900 ppm |
| Related compounds | |
| Related compounds |
Imidazole 2-Methylimidazole 4-Methylimidazole N-Ethylimidazole 1,2-Dimethylimidazole |