N-Methyl pyrrole sits among the cornerstones in the family of nitrogen-containing heterocycles. Its journey started in the nineteenth century when early organic chemists found interest in the aromatic qualities of pyrrole derivatives. Over time, as folks started tinkering more with methylation reactions, N-methyl pyrrole became a regular in the synthetic labs. Researchers in the mid-1900s were drawn to its role both as a building block and as a probe in mechanisms involving electron-rich aromatic rings. Some pivotal works in pharmaceutical chemistry also showcased its versatility not just as a curiosity, but as a scaffold for more ambitious molecular designs. Over the years, demand for derivatives of pyrrole only grew, especially as industries found new ways to engineer materials and fine chemicals.
N-Methyl pyrrole, a colorless to pale yellow liquid, makes its mark in the toolbox of chemists working on both small-scale syntheses and heavy-duty industrial projects. It’s not a household name, but it runs quietly behind the scenes in a host of applications: conducting polymers, anti-corrosion coatings, and even pharmaceutical intermediates. Chemically, N-methyl pyrrole distinguishes itself from plain pyrrole with a single methyl group tacked onto the nitrogen—an adjustment that pushes its properties just enough to open many new doors in reactivity. The slight change allows for increased stability and makes it a useful partner in reactions that form other complex molecules or materials.
At the bench, N-methyl pyrrole shows up as a low-viscosity, highly mobile liquid. Its boiling point hovers around 105-110°C. That means it can be distilled without breaking down, a convenient property for purification. Unlike pyrrole, the methyl substitution bumps its basicity down a notch—the lone pair on nitrogen spends more time with its alkyl partner and less time at play with strong acids or bases. The vapor carries a noticeable, sharp odor, and the liquid mixes well with most organic solvents, including acetone and ether. Folks handling it notice that it stains quickly in air, as oxygen-induced oxidation darkens its clear appearance within hours if not protected; this reminds everyone in the lab that thoughtful storage matters a lot.
Product quality for N-methyl pyrrole often gets defined by purity—most commercial bottles specify 98% or higher, with residual water content and trace impurities (like unreacted pyrrole or methylation byproducts) measured in parts per thousand or even lower. Labels list these values alongside recommended safety warnings, hazard categories, and relevant registration numbers. Shipping containers always come sealed under inert gas to avoid the rapid oxidation problem, and any supplier aiming at quality assurance will mention batch number and production date to back up traceability. Experienced users know to check certificates of analysis, since stability can slip if suppliers ignore handling guidelines.
The classic synthesis relies on N-alkylation. Here, pyrrole reacts cleanly with methyl iodide (or methyl sulfate in older routes) under mild conditions. I’ve seen setups using both phase transfer catalysis and direct addition, but the industry prefers methyl iodide for reliability and clean separation. Those who want greener ways have played with dimethyl carbonate or even biomimetic methyl donors, but conversion rates and cost still can't match traditional ways. Purification happens by distillation under reduced pressure, protecting product from oxygen, and removing polar byproducts using aqueous washes. The ease of this process plays a big role in the widespread adoption of N-methyl pyrrole as a synthetic intermediate.
N-Methyl pyrrole takes a back seat in electrophilic aromatic substitution compared to its parent pyrrole, thanks to the less active nitrogen, but it still reacts well with halogens and some acylating agents under controlled conditions. This makes it easier to stick alkyl, acyl, or even halogen groups onto its ring, bypassing complications seen with more reactive pyrroles. Reductive alkylations and arylations expand the structural scope, which is especially valuable in drug research. For polymers, N-methyl pyrrole steps up as a monomer in the chemical or electrochemical synthesis of polypyrrole materials. Simple oxidizers create linear chains that serve as conductive or antioxidative coatings—these kinds of reactions set the stage for some bold advances in electronic materials over the last few decades.
N-Methyl pyrrole shows up on chemical lists under several other names, including 1-Methylpyrrole, N-Methyl-1H-pyrrole, and methylpyrrole. In industrial catalogs, you’ll sometimes find it under trade numbers or product codes, but chemists tend to go with the simplest option. Its structural formula (C5H7N) or molecular identifiers like CAS number 96-54-8 ensure clarity in regulatory paperwork and when ordering from suppliers. Thanks to its defined usage in the chemical sector, confusion rarely crops up, as long as folks check both names and numbers when handling procurement or compliance documents.
Safety matters a great deal whenever liquid N-methyl pyrrole is in the lab. The sharp odor warns that vapor exposure isn’t something to ignore, so good fume hoods become non-negotiable for bulk handling or synthesis. Gloves and protective eyewear guard against skin and eye irritation reported after even minor spills. Storage always happens away from sources of ignition—the flash point clocks in under 30°C, so accidental ignition stands as a real concern in hot zones. Regulations in Europe, the US, and China classify it as a hazardous substance, which means users follow strict record-keeping and accident protocols. Disposal routes usually involve solvent incineration rather than standard drain treatment, out of concern for aquatic toxicity and persistence. Even for small amounts, local fire codes advise flammables cabinets and spark-proof equipment to reduce workplace risks.
N-Methyl pyrrole holds an important spot as a specialty intermediate. Pharmaceutical companies seek it for weaving together complex nitrogen-containing molecules, sometimes as a masking group in synthesis, other times building up new heterocyclic rings that serve as drug scaffolds. In the world of conductive materials, both academic and commercial research rely on it to make polypyrrole derivatives; the methyl group controls conductivity while ensuring thermal stability. Specialty coatings benefit as well, especially in anti-corrosion treatments for metals and electronics. Even some flavors and fragrances tinkered with its analogues, exploring bioactive potential. The agricultural sector took notice, too—as a step in the path toward certain crop protection agents, N-methyl pyrrole finds its way into the world’s fields, not merely the laboratory.
Research circles keep returning to N-methyl pyrrole for both curiosity-driven and mission-critical projects. While some work focuses on tweaking its aromatic backbone for new reactive patterns, others concentrate on fine-tuning polymer synthesis. Researchers explore its role in neural interfaces, thanks to polypyrrole’s use in biocompatible electrodes. Pharmaceutical advances often look at modifications for new fully synthetic routes to alkaloid-like compounds. Among green chemists, the push focuses on finding new methylating agents that don’t leave behind problem byproducts or require hazardous solvents. Each year brings refinements—better yields, smarter process integration, and expanded safety protocols. The momentum hasn’t slowed, since companies see real impacts from even small improvements in how N-methyl pyrrole gets made or modified.
N-Methyl pyrrole itself lands on the list of "use with care." Animal tests show moderate acute toxicity when ingested or inhaled, with symptoms affecting the liver and nervous system at high doses. Mutagenicity assessments usually point to low genotoxicity, but data gaps remain, especially for chronic low-level exposure. Environmental studies suggest that its breakdown products don’t linger long, but spills threaten aquatic life and lead to oxygen depletion. Occupational safety teams advocate for strict airborne concentration limits, and suppliers must follow REACH and TSCA regulations in packaging and labeling throughout Europe and North America. The research community continues to push for additional studies—the data so far confirm it belongs in well-controlled settings, and ongoing monitoring helps head off hazards associated with changing large-scale production methods.
Looking forward, N-methyl pyrrole stands ready to anchor new trends in both materials science and medicinal chemistry. Polymer researchers see growing interest in advanced conductive blends for everything from energy storage to wearable electronics, areas where this compound’s unique reactivity unlocks design flexibility. The pharmaceutical sector continues to invest in derivative libraries, betting on the structure’s adaptability to meet pressing needs in drug discovery. As regulatory pressures mount on solvent use and workplace safety, the pipeline fills with process chemists striving for cleaner, safer methylation strategies and less wasteful purification methods. Across the board, innovation hinges on collaboration between firms, universities, and regulatory bodies to blend real-world know-how with evolving science. Most agree the story of N-methyl pyrrole isn’t finished; it looks set to remain a quiet driver of progress wherever chemists need flexible, well-understood building blocks.
Most people have never heard of N-Methyl Pyrrole, but it helps shape products we bump into every day. Chemists and engineers love this little molecule for a reason—it’s not showy, but once you see what it can do, it’s hard not to respect its spot in the lineup of essential building blocks.
Many modern devices run on high-performance materials. N-Methyl Pyrrole steps up as a core ingredient in the making of polypyrrole, a type of conductive polymer. This polymer finds a purpose in flexible electronics, touch-sensitive devices, and some smart textiles. It brings this mild, reliable conductivity—just enough to make a circuit work without the bulk or fragility of metal. For someone who has tinkered with flexible circuit boards in a high school robotics club, finding out the origins of that conductive layer drove home how much chemistry sits behind technology.
A simple tweak in its molecular shape—here, swapping a hydrogen for a methyl group—means N-Methyl Pyrrole creates variations on polypyrrole that let engineers tailor the flexibility or conductivity. It’s a bit like using different flour for bread and cakes. Both are flour, but with a small change in texture and behavior.
Chemistry classes often talk up pyrrole rings for their importance in living systems—think hemoglobin and chlorophyll. N-Methyl Pyrrole offers a shortcut for pharmaceutical scientists looking to build more complex molecules. It shows up in the process of creating medicines, including some anti-inflammatory and antimicrobial drugs. Adding a methyl group can make a molecule more stable or easier for the human body to use.
People working in perfume labs also turn to N-Methyl Pyrrole. Its structure forms part of the backbone for many aroma molecules that mimic the scent of freshly roasted coffee or sweet tobacco. A small change at this chemical level changes how a fragrance lingers or changes through the day. It’s proof for anyone sensitive to smells—like me—how the tiniest chemical tweak can shape an entire experience.
Some of the brightest blues, greens, and even black dyes in textiles and printing come from compounds that started out in a chemistry lab with N-Methyl Pyrrole. The molecule pops up when making advanced pigments for plastics, paints, and even optoelectronic materials. Researchers push these boundaries every year, using pyrrole derivatives to make colors that last longer and waste less energy. Anyone who has seen a favorite shirt fade too soon can appreciate the effort to make colors more stable.
N-Methyl Pyrrole doesn’t get headlines, but anyone working with it needs to stay sharp about safety. It’s flammable and can irritate the skin and lungs. Labs and factories handle it with gloves and good ventilation, backed by clear data on its properties from groups like the European Chemicals Agency and the CDC. Safety sheets recommend proper storage and careful monitoring, helping to protect the people making our modern science possible.
With the world chasing greener chemistry and safer workplaces, there’s always a push for makers to streamlining production, reduce waste, and control emissions. Paying attention to these details matters—not just for lab workers, but for anyone relying on safer, cleaner products.
N-Methyl Pyrrole shows up in labs thanks to its role in making complex organic compounds. It’s a colorless to pale yellow liquid, giving off a strong, sharp odor. Some chemicals cause hazards you don’t spot right away, and N-Methyl Pyrrole stands among them. In my days working with solvents and reagents, those bottles with warning labels always reminded me to think before acting. Safety matters a lot more after seeing what can happen when it’s ignored.
Take air seriously. Vapors from N-Methyl Pyrrole aren’t something you want in your lungs. Work in a chemical fume hood or at least a very well-ventilated spot. Flimsy excuses, like “I’ll just be quick,” lead to bad habits. Good ventilation stays non-negotiable.
Skin contact brings its own risks. This compound can get through latex. Nitrile gloves serve better, and double-gloving brings peace of mind if you’re handling it for more than a minute or two. Spills always surprise, so sleeves pulled down and a lab coat add another layer of protection.
As for eyes, splash goggles win over regular glasses. Even a splash can burn or do damage you’ll regret. Face shields earn their place for bigger jobs or when pouring larger amounts.
Quick action limits the harm. If you breathe in vapors, get out into fresh air. Don’t try to shrug off dizziness or coughing. If the skin gets hit, rinse with water for at least fifteen minutes and strip away contaminated clothes right away. For eyes, use an eyewash station and keep rinsing. Even if pain backs off, go see a doctor—delayed reactions can occur.
N-Methyl Pyrrole doesn’t play well with heat or open flames. It’s flammable, so keep it away from ignition sources. Store it in tightly-sealed glass bottles, away from sunlight, in a cool spot. Label everything with clear chemical names and hazard warnings. In shared spaces, “mystery bottles” invite accidents.
Every lab spill brings a mess, but N-Methyl Pyrrole’s vapors mean you can’t just mop up and leave. Use absorptive materials—vermiculite or sand—not rags. Gather up the spill, and put all cleanup materials in a proper hazardous waste container. Avoid drains. That fluid finds its way into water faster than some expect.
Rules help, but real safety grows from training and attitude. New researchers, students, or employees need walk-throughs and mentoring. People make mistakes, but good habits come from experience. Authorities like OSHA lay out rules, but I’ve seen that clear-headed peer reminders stop more accidents than posters on a wall.
The EPA lists N-Methyl Pyrrole among chemicals that threaten water and soil if released. This signals that safe handling isn’t about personal injury alone. Chemical hygiene protects both people and the wider community.
Working with N-Methyl Pyrrole gets routine for some. It pays to treat every transfer, pour, or measurement as something new. PPE, ventilation, and storage turn risk into safety. Teaching these habits means less panic, fewer injuries, and a safer workplace for everyone.
N-Methyl pyrrole’s chemical structure stands out among simple organic compounds. At first glance, it looks like a five-membered ring where four carbon atoms and one nitrogen make up the backbone. Swap one hydrogen on that nitrogen for a methyl group, and you end up with N-Methyl pyrrole. Chemists represent its molecular formula as C5H7N. That extra methyl group might seem like a minor tweak, but it changes both the molecule’s behavior and how it gets used in research and industry.
Adding a methyl group onto the nitrogen pulls N-Methyl pyrrole away from its parent, pyrrole. That single step brings about differences in electron distribution and alters its hydrogen bonding ability. Lab researchers learned early on that this means N-Methyl pyrrole won’t act quite like pyrrole in many types of reactions. In production environments, this matters because reactivity can make or break a process. Chemists appreciate how the methyl group blocks easy access to the nitrogen, which means this compound can serve as a test case for understanding selective reactions or be used to “mask” a reactive site in stepwise syntheses.
In my own experience with synthetic chemistry, I have seen how this simple five-membered ring and methyl group get put to work. Benzene and its cousins often get most of the classroom attention but working directly with N-Methyl pyrrole gives a different perspective. Its ring system holds aromatic character, a concept familiar to anyone who paid attention during organic chemistry, so it resists many of the reactions “ordinary” double-bonded rings undergo. The presence of the methyl on nitrogen stops proton swaps and blocks forming hydrogen bonds — a fact that wildlife researchers and pharmacologists take seriously not just in the lab, but in planning field work.
Beyond basic laboratory use, the story of N-Methyl pyrrole extends into plastics and medicine. In polymer science, this ring forms the backbone for certain conductive polymers. These materials turn up in flexible circuits and advanced sensors. Drug designers look at N-Methyl pyrrole and its analogues, searching for molecules which slip past enzymes or cross cellular barriers more easily. Often, a tiny change such as swapping in a methyl group tunes the molecule’s “fit” in a biological system.
The relatively simple structure of N-Methyl pyrrole makes for easy bench preparation, yet that same simplicity can hide safety challenges. The compound produces fumes that call for solid ventilation. Those handling it know enough to use gloves and work behind a fume hood. Waste goes to qualified collection since even small quantities can contribute to chemical load in groundwater if not disposed of carefully. It pays to take every step seriously, both for individual safety and community health.
Smarter chemistry starts with respect for chemical structure. Teams working in specialty polymers and new drug development could lean on green chemistry approaches to minimize waste and make sure every step adds value. I have seen labs succeed when they prioritize training and take the extra effort to confirm every step, whether for material reuse or waste treatment. The real reward comes as safer processes and better outcomes — not just on a spreadsheet, but in cleaner air and water around us.
Working in a lab or any place where chemicals circulate daily, the dangers behind a label aren’t just warnings—they are lessons learned from real mistakes. N-Methyl Pyrrole carries its own risks, like toxic fumes and reactivity, and they can land someone in trouble without the right approach. Breathing in the vapors or spilling a bit on bare skin reminds me of the importance of good habits and strong protocols.
Glass bottles with secure, chemical-resistant caps get the job done. I’ve seen cheap plastics swell or crack after repeated use, especially with chemicals that don’t play nice with polymers. Metal sounds safe, but corrosion has ruined enough samples that I avoid it. Every time I skip a proper label or a container looks cloudy or worn, I hear the voice of my old supervisor: “Replace it before you regret it.”
Cool, dry, and dark areas protect not just N-Methyl Pyrrole but also people working nearby. Any fluctuations in temperature—from sunlight or proximity to equipment that heats up—means volatility goes up, raising the chance for accidents. A storeroom in the corner of a building, shielded from windows and away from heat sources like radiators or ovens, cuts down most hazards. Humidity leads to condensation, and in my experience, water and reactive chemicals don’t mix well.
You don’t want to smell this stuff—good ventilation isn’t a bonus, it’s required. A ventilated cabinet or a fume hood with active fans prevents vapors from lingering. I’ve seen what happens with poor airflow: chemical odors travel, headaches start, and then the safety officers visit for an unplanned audit. Storing N-Methyl Pyrrole apart from acids, bases, and oxidizers helps prevent unexpected reactions. Stacking incompatible bottles together can turn a hot day into an emergency.
Some people look at routine checks as a chore. I see them as insurance. Caps loosen over time, labels fade, and tiny leaks build up unnoticed. Every few weeks, I check for shelf signs of crusts, stains, or odd smells. Once, a small unnoticed leak created a sticky patch under a bottle—caught in time, it meant a quick cleanup, not a facility shutdown. Replacing old stock stops embarrassment and risk from expired chemicals.
Lab safety drills used to seem pointless, until someone panicked over a dropped bottle. Real training sticks. New staff shadow experienced hands; we stop to explain why we wear goggles, use gloves, and keep spill kits ready. Folks learn that the best place for N-Methyl Pyrrole keeps it both accessible for work and safe from daily traffic. We don’t just rely on rules posted on the wall—shared experience and real stories carry more weight.
Refreshing staff about safety doesn’t need red tape or endless meetings. Simple posters, quick quizzes, and guided walkthroughs do more to reinforce good storage habits. Investment in solid storage infrastructure, modern fume extraction, and supply of compatible containers pays off in reduced risk and smoother audits. It’s not just about ticking a box—it saves time, money, and, sometimes, a career. Experience is a tough teacher in chemistry. The goal is to learn from it before mistakes get the chance.
Every time pharmaceutical labs design new drugs, they face the challenge of finding the right building blocks. N-Methyl Pyrrole acts as one of those key ingredients. Medicinal chemists like it because it provides a versatile scaffold for developing compounds that target a wide range of conditions. During research, I’ve seen this compound used as a stepping stone for drugs designed to treat cancer, bacterial infections, and inflammation. The nitrogen-containing ring system in N-Methyl Pyrrole mimics structures often found in natural products, which lets researchers explore new ways to block key enzymes or interact with receptor sites. Multiple peer-reviewed studies point to its presence in experimental molecules that end up as drug leads. Researchers at the intersection of chemistry and medicine need reliable intermediates like this one to keep the innovation pipeline moving.
You won’t find many stories about plastics in sci-fi movies, but in real-world labs, materials science has become just as exciting. N-Methyl Pyrrole forms the backbone of some high-performing conducting polymers, such as polypyrrole. These plastics don’t just sit in an outlet cover; they can carry electrical current, opening up possibilities for flexible electronics, lightweight batteries, low-cost sensors, and even neural interface devices. I’ve seen research groups use polypyrrole blends to create smart fabrics and wearable sensors, aiming to revolutionize health monitoring. The ability to control electrical conductivity without relying on metal wiring means scientists can engineer lighter, more adaptable products.
In a world where more devices connect to the Internet of Things, having safe and reliable polymer-based electronics always becomes more valuable. N-Methyl Pyrrole is one of the simple starting materials that makes this new generation of electronic materials possible.
Farming depends on chemistry far more deeply than people might realize. Crop scientists and agronomists look for ways to control pests while reducing harm to the environment. N-Methyl Pyrrole has a role in synthesizing certain herbicides and fungicides. Its compact structure allows chemists to build molecules that fit tightly with the enzymes in weeds and fungi, leading to more targeted action and fewer off-target effects. The trend toward precision agriculture only sharpens the need for advanced chemical ingredients. By designing more efficient active ingredients, N-Methyl Pyrrole supports higher crop yields and more resilient food systems.
Beyond blockbuster uses, factories turn to N-Methyl Pyrrole to fine-tune specialty chemicals, dyes, and corrosion inhibitors. Chemists in the textile and printing industries value it for its reactivity, using it to help develop dyes with brighter colors and better wash resistance. In some labs, I’ve seen it used as a chemical intermediate for making solvents and as part of the process that builds complex molecules step by step.
Like many useful industrial chemicals, N-Methyl Pyrrole calls for respect during handling. It can be tricky to store due to its sensitivity to light and air. To manage risks, suppliers are moving toward safer packaging and clearer handling instructions. In my experience, regular training and robust engineering controls help reduce exposure for workers. Companies seeking greener chemistry solutions can look into using N-Methyl Pyrrole more efficiently, recycling it where possible, and adopting safer substitutes on a case-by-case basis. With rising attention to environmental stewardship, industry leaders need to stay engaged with new research into sustainable production methods and safer end-of-life disposal.
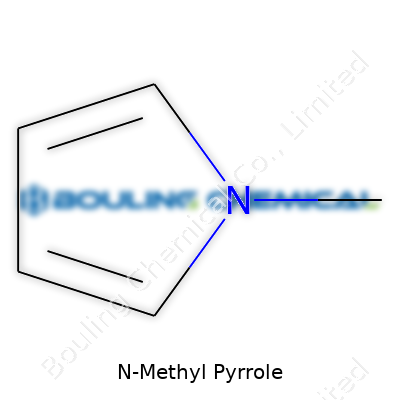
| Names | |
| Preferred IUPAC name | 1-Methyl-1H-pyrrole |
| Other names |
1-Methylpyrrole N-Methyl-1H-pyrrole N-Methylpyrrole 1H-Pyrrole, N-methyl- |
| Pronunciation | /ɛn-ˈmɛθɪl pɪˈroʊl/ |
| Identifiers | |
| CAS Number | 120-94-5 |
| Beilstein Reference | 1366056 |
| ChEBI | CHEBI:51109 |
| ChEMBL | CHEMBL14220 |
| ChemSpider | 62644 |
| DrugBank | DB01878 |
| ECHA InfoCard | 13e2e1e6-613e-4a03-8aad-7e6a4b2bf36a |
| EC Number | 613-040-00-8 |
| Gmelin Reference | 8719 |
| KEGG | C06427 |
| MeSH | D051485 |
| PubChem CID | 7858 |
| RTECS number | UY2975000 |
| UNII | N5M4IFA8FZ |
| UN number | UN2733 |
| Properties | |
| Chemical formula | C5H7N |
| Molar mass | 81.12 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | Aromatic |
| Density | 0.944 g/mL at 25 °C |
| Solubility in water | slightly soluble |
| log P | 0.85 |
| Vapor pressure | 2.9 mmHg (20°C) |
| Acidity (pKa) | 23.3 |
| Basicity (pKb) | pKb = 13.8 |
| Magnetic susceptibility (χ) | -26.7×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.501 |
| Viscosity | 1.005 cP (20°C) |
| Dipole moment | 1.74 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 183.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -23.0 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2950 kJ/mol |
| Pharmacology | |
| ATC code | N05CM18 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02, GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H312, H332 |
| Precautionary statements | P261, P280, P304+P340, P312, P501 |
| NFPA 704 (fire diamond) | 2-2-0 |
| Flash point | 41°C |
| Autoignition temperature | 315 °C |
| Explosive limits | 1.8–10.6% |
| Lethal dose or concentration | LD50 oral rat 1083 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat >5110 mg/kg |
| NIOSH | NIOSH: QP9450000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for N-Methyl Pyrrole: Not established |
| REL (Recommended) | 800 ppm |
| IDLH (Immediate danger) | IDLH: 75 ppm |
| Related compounds | |
| Related compounds |
Pyrrole 2-Methylpyrrole Pyrrolidine N-Methylindole N-Methylpyrrolidone |