Chemists started experimenting with piperidine rings well into the 20th century, and the idea to tweak these molecules for better performance grew year after year. The modification at the fourth position—where bromine takes the spot—and methylation of the nitrogen, followed naturally from broader research into psychoactive wires, specialty catalyst design, and pharmaceutical ambitions. Synthetic chemistry always strikes a balance between ambition and practicality, and once researchers noticed the effects of bromine substitution, labs in Europe and North America saw benefit not in just tweaking structures but in favoring those that proved reliable, controllable, and relatively easy to produce. In the context of rapid changes in medicinal chemistry, this compound attracted new attention because it provided versatility for exploring novel therapeutic scaffolds or building blocks for material science and beyond.
N-Methyl-4-Bromopiperidine doesn’t sit on most chemists’ lips unless they browse catalogues for research intermediates, but for the right project, it’s invaluable. It treats structural rigidity not as a limitation but as an opportunity, finding roles in alkaloid analogues or specialty synthesis for small batches in the pharmaceutical pipeline. Its moderate stability and straightforward structure make it attractive in commercial research portfolios, which seek out compounds with clear, modifiable backbones and enough biological or industrial curiosity to keep testing new pathways. This is a molecule that quietly supports innovation rather than grabbing headlines.
Its chemical formula is C6H12BrN, which packs the essential basics: a piperidine ring, a bromine atom at the 4-position, and a methyl group attached to the nitrogen. As an oil or low-melting-point solid, its physical presence is unremarkable, but in practice, handling often requires careful ventilation thanks to its volatility and amine odor. It dissolves in common organic solvents, responds predictably to acid and base, and usually arrives as a pale-yellow oil. With a molecular weight around 194 g/mol and a boiling point near 220°C, it finds use at room temperature if properly sealed and stored, steering clear of water-rich environments to prevent hydrolysis or decomposition over time.
Any reputable chemical supplier labels N-Methyl-4-Bromopiperidine with at least 98% purity, which meets research and preclinical needs. On bottles, hazard pictograms warn of acute toxicity and environmental effects. Labels must list the CAS number—62794-48-7—along with molecular formula, lot number, production date, and storage instructions, usually calling for cool, dry conditions. Material Safety Data Sheets (MSDS) lay out the expected precautions, and packaging commonly involves amber or opaque containers to cut exposure to light, which can gradually alter sensitive organic compounds.
The go-to method involves starting from piperidine, which reacts with methylating agents (like methyl iodide or dimethyl sulfate) under base conditions to slip in the methyl group. After isolating N-methylpiperidine, chemists selectively brominate the 4-position with N-bromosuccinimide (NBS) or bromine in an inert atmosphere. Temperature control is crucial; too hot, and side reactions crop up—too cold, and yields dip. Work-ups mean glassware gets a strong wash with non-chlorinated solvents, followed by vacuum distillation or crystallization. Laboratories that chase higher yields lean on automated batch or flow technologies, minimizing reaction time and improving batch-to-batch consistency.
The real utility comes from the chemical flexibility. The bromine acts as a leaving group, opening up cross-coupling reactions or nucleophilic substitution. In medicinal chemistry, swapping the bromine with nucleophiles introduces functional groups tailored for specific receptors. For materials science, the piperidine ring holds up against tough conditions, providing a staging ground for more elaborate synthesis: couple with aryl boronic acids for Suzuki couplings, or turn to reductive amination to modify the nitrogen further. Bromopiperidines like this one have helped researchers design sharper CNS drugs and advanced agrochemical candidates, supporting new families of molecules with unique activities.
Catalogues list this compound under several names, including N-Methyl-4-Bromopiperidine, 1-Methyl-4-Bromopiperidine, and 4-Bromo-N-methylpiperidine. Each signals the same skeletal framework, so long as the methyl lands on the nitrogen and the bromine dots the fourth carbon. Specialty suppliers may invent code names or catalogue numbers, streamlining internal demand for custom batches. For regulatory tracking, the CAS number remains the most reliable way to avoid confusion across borders.
N-Methyl-4-Bromopiperidine demands respect and good handling. Gloves, goggles, and fume hoods should always make appearances before anyone cracks open a fresh bottle. The compound’s volatility can sneak up during transfers or reactions, leading to headaches or nausea on chronic exposure. Some nations flag it for precurson control under narcotics legislation, forcing additional paperwork or restricted access. Storage near acids or open flames brings avoidable risk: decomposition tails off bromine vapors, which are harder to scrub from lab air. Standard industrial hygiene—quick spill cleanup, dedicated disposal streams, and well-maintained ventilation—serves as the baseline. Only trained staff should run distillations or scale-up reactions in pilot labs, and emergency showers or eyewash stations need to stand close to each work station.
Most demand for N-Methyl-4-Bromopiperidine centers in the research lab: medicinal chemists design new CNS-active compounds, and its ready bromine supports easy substitution. Material scientists explore piperidine skeletons for advanced polymers, anti-corrosive additives, or molecular probes. In some sectors, this compound plays a supporting role in agrochemical development, delivering a versatile aromatic precursor thanks to its ease of ring functionalization. Specialty contract research organizations (CROs) see regular calls for the molecule as a validated intermediate, delivering kilogram-scale batches to pharmaceutical or chemical firms curious about oddball analogs or wishing to fine-tune product performance.
R&D paces the journey for this compound. Pharmaceutical teams chase analogues—modifying the ring to eke out better binding or lower toxicity. Material science divisions test fresh monomers for next-generation plastics or resins, drawn to the predictable chemistry and strong backbone of the piperidine core. Academic settings join industry in hunting molecular targets, using this molecule as a scaffold or starting point. Real progress tracks improvements in reaction efficiency: not just higher yields, but milder conditions and greener solvents. A few research groups target chiral resolution, betting that finely tuned stereochemistry might unlock new pharmacological niches.
Lab workers report stinging eyes, irritated skin, and headaches when exposed too long or too carelessly. Toxicological studies remain limited—few chronic data sets track large animal models—but rat and mouse assays suggest moderate acute toxicity, calling for careful waste management and personal protective equipment. Environmental toxicity stands out: like other brominated organics, it runs toxic for aquatic life, demanding responsible disposal via incineration or certified waste streams. Regulatory pressures grow as green chemistry standards stiffen, nudging labs toward alternative halides or modifications that dial back risk without sacrificing synthetic value.
N-Methyl-4-Bromopiperidine holds its ground as new synthetic strategies emerge. Automated microfluidics could streamline batch production while cutting waste. Moves to swap out bromine for less hazardous leaving groups gather speed but must match the reliability and selectivity seen here. Pharmaceutical researchers, seeking faster paths to lead compounds, test it in fragment-based screening and as a launch point for CNS activity mapping. Environmental demands urge greener chemistry: solvent recycling, real-time monitoring, and push-button synthesis. Long-range, this molecule’s story could pivot on how nimbly it adapts to shifting regulatory, safety, and process requirements—bolting classic reliability to the demands of a leaner, cleaner chemical industry.
N-Methyl-4-Bromopiperidine stands out in the crowd of organic compounds because its name alone hints at a lot. The backbone here is piperidine—a six-membered ring with five methylene groups and a nitrogen atom. Chemists know piperidine rings as the flexible workhorses in both pharmaceuticals and material sciences. For N-Methyl-4-Bromopiperidine, two things jump out: a methyl group attaches to the nitrogen atom, and a bromine atom snaps onto the fourth carbon in the ring.
Picture it like this: start with the piperidine wheel, add a bromine at position four, and slap a methyl group on the nitrogen. The chemical formula reads C6H12BrN. For folks with an eye for chemical structures, you’d spot the nitrogen with its methyl group quietly perched at the top, while the bromine brings bulk at position four, pulling electronic effects across the ring.
Some might shrug and say, “So what? Another molecule.” But chemistry has taught me plenty about hidden connections. The shape and makeup of N-Methyl-4-Bromopiperidine matter to pharmaceutical chemists looking for that next antiviral or analgesic agent. The piperidine core shows up in big-name drugs like paroxetine and risperidone, except those have different groups in different spots. Change a group here, add a halogen there, and suddenly the substance acts differently in the body or the environment.
I’ve worked with halogenated compounds before—they tend to be reactive and versatile. Adding a bromine gives the molecule a kind of chemical “handle.” That bromine can switch places with other groups under the right conditions, which opens up synthetic routes to more complex targets. Much of what happens in drug discovery comes from tweaking functional groups and seeing how those changes ripple through.
Handling compounds like N-Methyl-4-Bromopiperidine brings its share of honesty to academic and industrial labs. Piperidines can veer into the territory of controlled substances or precursor chemicals. Some governments track these compounds tightly because they show up in both legal and illicit syntheses. Having worked in teaching labs and industry, I know people triple-check bottle labels and watch for regulatory changes. The responsibilities go beyond just wearing gloves or goggles—logging usage and disposal makes just as much sense as balancing a reaction equation.
One thing keeps coming up in conversations among researchers: better training and awareness. It’s not enough to know the chemical structure or to recognize the shape on paper. There’s a strong push toward better stewardship of chemicals with dual-use potential. Organizations like the American Chemical Society and various regulatory agencies publish regular updates, urging chemists to pay close attention.
Instead of just focusing on molecular diagrams, the spotlight shifts to education, safe handling policies, and transparent supply chains. For those trying to build the next generation of lifesaving compounds, a strong foundation starts by respecting both the chemistry and the wider impact each chemical brings.
Some see N-Methyl-4-Bromopiperidine simply as another building block. I see it as a chance for the next breakthrough—maybe a smarter synthetic route, maybe a cleaner reaction, or maybe just tighter security and awareness. The structure is simple enough, but its story unfolds in real-time, wherever science and responsibility cross paths.
Some compounds float through life’s backstage, doing the heavy lifting that never makes headlines. N-Methyl-4-Bromopiperidine is one of those. Cooks in lab coats? They know it for the key it can be toward unlocking bigger molecules, especially in the pharmaceutical world. Even if the name sounds like alphabet soup, its role is concrete. Every pill, patch, or bottle starts with steps like this, so digging into how this compound sees use helps everyone understand where modern medicine comes from.
Drug discovery leans on building blocks. N-Methyl-4-Bromopiperidine acts like a connector in chemical reactions. Scientists use it as what they call an intermediate—that's a substance that sits in the middle of a long string of steps, connecting the raw material stage to the active medicine. In practice, it allows chemists to add piperidine rings and nitrogen atoms to molecules. Those aren’t just science words. They change how drugs work in the human body by tweaking solubility or making drugs stay longer in the bloodstream.
No surprise, some antidepressants, cough suppressants, and antipsychotics trace their roots back through compounds made with this chemical. Piperidines in general show up often in treatments for brain chemistry or pain because their structure helps cross into tricky areas like the brain. My time sketching out molecules in college taught me that even tiny changes to these cores can make the difference between a blockbuster medicine and something that goes nowhere. N-Methyl-4-Bromopiperidine doesn’t get public attention, but without it, these breakthroughs probably would not happen as quickly.
Scale matters. A single researcher in a lab can test a compound with eye droppers and pipettes, but making enough for a clinical trial means pulling levers in huge reactors. N-Methyl-4-Bromopiperidine comes through not just because it works in small batches but because it holds up in big ones, too. Its reactivity and structure make it handy for introducing bromine atoms into chains. This kind of predictability only comes after plenty of hard-earned experience at the industrial level.
I’ve seen production lines grind to a halt waiting for quality intermediates to arrive. Sub-par chemicals can ruin weeks of work, burning time and money. A steady source of reliable N-Methyl-4-Bromopiperidine gives factories a way to cut down on these costly misfires.
Beyond drug companies, specialty chemical researchers use this compound as a launchpad into a whole category of piperidine derivatives. Those derivatives feed into everything from agricultural chemicals to new polymers. Chemistry isn’t just about textbook reactions—real labs deal with messy, unpredictable results. One thing that helped during my internship years: have robust safety protocols and solid sourcing. Handling reagents like this takes more than gloves and goggles.
Environmental concerns creep in, too. The bromine atom can cause trouble if people dispose of waste poorly or cut corners in production. Better tracking and more transparent supply chains would help push the industry to greener standards. Regulatory checks keep some of the worst sidelined, but pressure for responsible manufacturing stays essential.
No single compound changes the world alone. N-Methyl-4-Bromopiperidine works as a reminder that progress depends on the nuts and bolts behind the scenes. Supporting ways to make chemicals cleaner and more safely only ensures that promising molecules can do what they’re meant to—keep making real differences in labs and lives.
Anyone who has spent time in a chemistry lab can recall the tales of people who underestimated their reagents. N-Methyl-4-Bromopiperidine brings its own set of worries. This stuff doesn’t grow on trees—it comes with a punch. With its alkylating capability and bromine atom, it can irritate skin, eyes, and airways. You may not see the fumes, but they find you quick if you overlook proper handling.
No one starts working with reactive chemicals in shorts and flip-flops—not if they expect to stay out of the urgent care line. For N-Methyl-4-Bromopiperidine, long lab coats protect your arms, and goggles keep splashes off your face. Gloves—nitrile, not latex—matter more than ever. You might skip gloves for dish soap. Don’t skip them here. Respirators are worth the effort if dust or vapors are part of the job. If you ask around in any chemical supply room, there’s usually a story about someone dodging basic PPE and paying the price.
Fume hoods aren’t just fancy shelves with windows. They pull away the vapors you can’t see. Breathing in any volatile amine isn’t fun. Piperidine derivatives, once inhaled, seem to linger. Running experiments in open rooms sets the scene for someone to get a beating headache—or worse. No one enjoys spending the afternoon in the safety office filling out incident reports.
I’ve knocked over enough bottles to know how fast things can go wrong. If a spill happens, step back and let the fume hood do the clearing. Covering a puddle with absorbent pads stops it from spreading. Disposing of them means using designated bags or containers. Work areas need eye wash and safety showers within reach, and every lab rat should know how to use them without fumbling. Quick rinsing can mean the difference between five minutes’ discomfort and a trip to the hospital.
Sharpyed labels lose their writing quick. Printed, chemical-resistant labels list more than the name—they give hazards and handling tips, too. I’ve seen students confuse similar bottles, and it’s only luck that nobody mixed incompatible chemicals by mistake. It pays off to keep logs and store these materials by their hazard class, never jammed onto crowded shelves beside water-reactive or oxidizing agents.
There’s always the temptation to flush waste down the drain or toss it in the general trash. Bad move for anything containing bromine or complex nitrogen compounds. Hazardous waste bins are there for a reason. Teams pick up those containers and take them to incinerators or secure facilities. Ignoring these steps can earn fines or put neighbors at risk.
One-off safety briefings don’t cover everything. Refreshers stick when trainers share stories of close calls and mistakes. In workplaces where new faces cycle in often, it helps to assign mentors who spot risk before it happens. It’s not about following rules for rules' sake—it’s about keeping everyone ready to leave the building in the same shape they walked in.
No worker exists in a bubble. I’ve worked with teams who watch out for each other. If someone looks lost, a quick check-in means more than a lecture. Creating that culture—where folks say something when they see something—keeps the material from turning into a headline about what went wrong rather than what was prevented.
Nobody in the lab asks, “Is that good enough?” just for the fun of it. Every time I crack open a new bottle, knowing the purity matters. With N-Methyl-4-Bromopiperidine, the story feels the same as with most reactive chemical building blocks—every decimal point matters. Analytical reports from reputable suppliers usually show purity above 98%, using methods like GC-MS or NMR to back it up. Even at face value, that 98% should prompt folks to dig deeper. Impurities have a way of transforming a reaction outcome, usually not for the better. Especially in medicinal chemistry or pharma contexts, even a tiny percentage of an unknown or poorly documented impurity can spell bigger headaches than anyone wants.
Most spec sheets include purity percentages, identification by NMR and IR, a description of appearance—almost always a colorless to pale yellow liquid—and counts of moisture and residual solvents. For this compound, water content stays below 0.5%, since excess moisture tends to muck up reactions. Heavy metals? Anything under 10 ppm is what most chemists look for. Most stocks come with a boiling point listing around 80–82°C at reduced pressure, and a density of about 1.41 g/cm³. I’ve seen labs ignore these data points and end up chasing down ghost problems in their syntheses, like unexplained side-products or a batch that refuses to crystallize.
My early days in research didn’t leave much room for cutting corners. Anyone who’s stayed late, watching a column run for hours, knows the pain of discovering root-cause issues traced back to an impure starting material. If the N-Methyl-4-Bromopiperidine was below 98%, side-reactions increased or yields dropped in multi-step syntheses. On one occasion, an extra peak on the NMR pointed to an impurity that stalled a full week of progress. No scientist wants to repeat steps for a solvable problem.
My go-to move before buying: ask suppliers directly for recent batch analysis, not just old COAs floating around online. The year a pandemic hit, labs with inconsistent supply chains saw a spike in variable purity levels. People double-checked every new drum. Most solid vendors offer LC-MS or NMR data, but it surprises me how often labs skip reviewing this before use.
Choosing the right supplier means more than opting for the cheapest. Reliable companies invest in quality assurance and keep their processes sharp. I’ve seen friends order from lesser-known outfits and receive materials reeking of solvent or colored by decomposition—not exactly confidence-inspiring.
It’s easy to blame a bad reaction on the technique, but often, a batch of subpar starting material ruins even the best methods. Standardizing purity assessment and open reporting could prevent these problems. In my experience, pushing vendors to explain their testing protocols and demanding full spectroscopic data up front keeps everyone honest and helps avoid wasted resources later. I favor labs that not only expect these demands but actually welcome them. Adoption of rigorous purity checks has started to gain ground, which tells me more of the field understands what’s at stake when corners get cut.
People who handle N-Methyl-4-Bromopiperidine or similar reagents know one truth: start with the highest purity, demand full transparency, and double-check what comes in. Every project depends on it. Experience proves chasing high standards up front beats any attempt to rescue a project down the line.
N-Methyl-4-Bromopiperidine sounds like another obscure chemical, but ignoring its handling rules could invite all sorts of trouble. Anyone who has worked in a lab with reactive chemicals knows accidents rarely start as big events. Often, a cracked container or the wrong kind of box creates a situation that escalates fast. For compounds like this, setting up the right storage and shipping approach saves more than merchandise—it can protect people and entire facilities.
From experience, improper storage of volatile reagents like this creates a domino effect. Leaky lids or exposure to sunlight ends up contaminating everything nearby, and the cleanup leaves lasting headaches. For N-Methyl-4-Bromopiperidine, keep temperature consistent—ambient room temperature usually works, away from direct sunlight or any source of heat. Humidity speeds up degradation. A desiccator or moisture-barrier cabinet stops damp air from getting in and helps avoid sticky messes or possible chemical breakdown.
Some forget the risk of storing incompatible chemicals together. Mixing halogenated reagents with oxidizers or acids in the same cabinet creates opportunities for runaway reactions. Segregate based on reactivity—I once had to help evacuate a research wing after a storage mishap blended two otherwise “stable” products. Keep N-Methyl-4-Bromopiperidine away from open flames or sources of ignition, since many piperidines catch fire or release noxious fumes if overheated.
Glass bottles with chemically resistant stoppers work best for small amounts, but anyone dealing with bulk shipments should switch to high-density polyethylene (HDPE). I’ve seen glass shatter at the worst possible times, turning a normal delivery into a biohazard event. Always double-bag containers and seal everything tight to block escape of fumes. Use clear labels—not just for safety, but for compliance if you’re dealing with regulated substances or crossing borders.
Secondary containment, such as a sealed drum or plastic bin, keeps accidental spills from spreading. In one company, we once caught a spill in a secondary tray that could have damaged thousands in equipment. Routine checks for cracks or leaks beats even the fanciest alarm system.
Shipping dangerous goods means paperwork and extra preparation. The United Nations and DOT assign codes and categories for a reason. N-Methyl-4-Bromopiperidine, classified as a hazardous material, requires special outer packaging, cushioning to prevent shock, and airtight containers. Air shipments demand UN-certified packaging and the right hazard labels; ground and sea have their own requirements. Skipping documentation risks fines or worse.
I’ve seen a container held at customs for weeks because a supplier underestimated paperwork. Working with a courier experienced in hazardous material transport helps. These specialists know the latest rules and handle emergencies on the spot. Insist on temperature tracking and shipment monitoring if the cargo will see rough conditions.
Personal experience taught me most spills and accidents come down to human error—misplaced confidence, rushed handling, and poor training. Always give staff real-world demonstrations on spill kits, PPE, and emergency procedures. Encourage a culture where people double-check rather than guess. If anything seems off, stop and reassess storage or shipping before it’s too late.
With chemicals like N-Methyl-4-Bromopiperidine, safe storage and correct shipping keep the lab running instead of scrambling for respirators and damage control. A little attention up front, the right container, and solid training save much more than just money. Safety puts everyone at ease, making the real work possible.
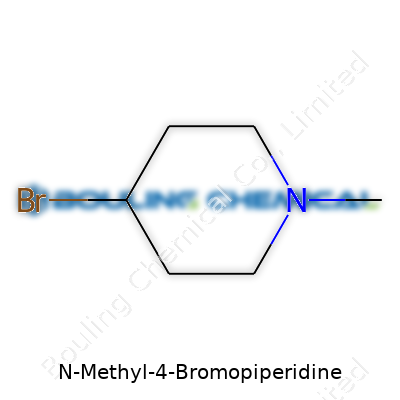
| Names | |
| Preferred IUPAC name | 1-methyl-4-bromopiperidine |
| Other names |
1-Bromo-4-methylpiperidine 4-Bromo-1-methylpiperidine N-Methylpiperidin-4-yl bromide |
| Pronunciation | /ɛn-ˈmɛθɪl-fɔːr-ˈbroʊmoʊ-pɪˈpɛrɪdiːn/ |
| Identifiers | |
| CAS Number | 52905-35-8 |
| 3D model (JSmol) | `CN1CCC(CC1)Br` |
| Beilstein Reference | 3918733 |
| ChEBI | CHEBI:131356 |
| ChEMBL | CHEMBL1974021 |
| ChemSpider | 152116 |
| DrugBank | DB04350 |
| ECHA InfoCard | 100_151_013 |
| EC Number | 620-478-7 |
| Gmelin Reference | 105093 |
| KEGG | C14351 |
| MeSH | D03721 |
| PubChem CID | 12468073 |
| RTECS number | TM3158000 |
| UNII | 25C6R8P4KS |
| UN number | UN2810 |
| Properties | |
| Chemical formula | C6H12BrN |
| Molar mass | 190.08 g/mol |
| Appearance | Colorless to light yellow liquid |
| Odor | Amine-like |
| Density | 1.36 g/cm³ |
| Solubility in water | soluble |
| log P | 0.83 |
| Vapor pressure | 0.3 mmHg (25 °C) |
| Acidity (pKa) | 11.2 |
| Basicity (pKb) | 3.56 |
| Magnetic susceptibility (χ) | -73.06e-6 cm³/mol |
| Refractive index (nD) | 1.495 |
| Viscosity | 29 mPa·s (20 °C) |
| Dipole moment | 3.15 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 340.6 J·K⁻¹·mol⁻¹ |
| Pharmacology | |
| ATC code | N04BX |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS06 |
| Signal word | Danger |
| Hazard statements | H301 + H311 + H331: Toxic if swallowed, in contact with skin or if inhaled. |
| Precautionary statements | P210, P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-3-0-W |
| Flash point | 67 °C |
| LD50 (median dose) | LD50 (median dose): 107 mg/kg (mouse, intraperitoneal) |
| NIOSH | BX8575000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.1 ppm |
| IDLH (Immediate danger) | IDLH: Not established |
| Related compounds | |
| Related compounds |
4-Bromopiperidine N-Methylpiperidine Piperidine N-Methyl-4-chloropiperidine N,N-Dimethyl-4-bromopiperidine |