Chemists built the reputation of N-Hydroxysuccinimide, or NHS, not overnight, but through determined steps in the labs of the 20th century. After its synthesis in the mid-20th century, NHS entered organic chemistry as a crucial player for amide bond formation. This proved to be a breakthrough for making peptides, a necessity in medicine and research. By coupling carboxylic acids with amines in the presence of carbodiimides, several teams realized NHS boosts both yield and reliability, making it a staple for researchers developing new therapeutics or diagnostic tools. From early frustrations with messy side products, the arrival of NHS meant labs could count on more consistent reactions. Over the decades, its reliability cemented its place in both academic and industrial chemistry.
N-Hydroxysuccinimide stands out for its versatility. Chemists value its white, crystalline appearance, which hints at stability and quality. As someone who’s worked at a benchtop hustling through protein labeling tasks, grabbing a vial of NHS signals a straightforward synthesis day. Its commercial forms typically arrive tightly sealed, dry, and measured out for convenience, no guesswork involved. Thanks to attention to consistency, NHS now appears in catalogues of nearly every big chemical supplier, available with different purities and packaging sizes for both small bench routines and full-scale production.
NHS’s chemical structure—a five-membered succinimide ring with a hydroxyl group—gives it just the right blend of stability and reactivity. Its molecular formula is C4H5NO3, and it weighs in at around 115.09 g/mol. In practice, it readily dissolves in polar organic solvents like DMF, acetonitrile, or DMSO, but is less compatible with plain water because of slow hydrolysis. You notice an earthy odor when opening a fresh container. It remains stable under cool, dry conditions; exposure to moisture triggers unwanted reactions, so labs keep it tightly capped or stored under nitrogen. NHS doesn’t handle extreme pH or persistent light well, as it’s known to degrade.
Reputable suppliers specify minimum purity, usually upwards of 98%. Labels give the CAS number (6066-82-6) and details like batch number, expiration date, and handling instructions. Data sheets outline solubility, melting point (around 96–98°C), and recommended storage conditions: below 4°C and in airtight containers. For larger batches or regulated environments, labels include hazard and precautionary statements according to GHS standards, listing irritant warnings and proper protective equipment—gloves and goggles are no joke here. Shipping documents flag NHS as a chemical that merits careful handling. Regulatory scrutiny drives manufacturers to improve both transparency and quality, so end users know exactly what they’re working with.
Industrial production of NHS typically kicks off with succinic anhydride reacting with hydroxylamine. The reaction takes place in controlled, cooled vessels because the exotherm can run wild if left unsupervised. Once the mixture reacts fully, purification steps follow, from solvent washes to recrystallization. These steps keep impurities, like unreacted succinic anhydride or side products, out of the final product. The process keeps to industry standards for safety, but even in smaller lab syntheses, careful temperature monitoring and slow addition rates matter. Quality relies on precise control here, and even a slip—like adding hydroxylamine too fast—can cause dangerous decomposition or poor product yield.
NHS’s true value comes from its chemistry. In peptide and protein work, activating carboxylic acids using NHS makes the difference between a poor yield and a crisp, successful reaction. NHS esters are much more reactive than free acids, so attaching them to biomolecules feels almost effortless to those familiar with finicky amide coupling reactions. NHS easily forms esters with carboxylic acids in the presence of carbodiimides, producing a shelf-stable intermediate. These esters then hook up with primary amines, linking peptides or antibodies with dyes or drugs. In the context of developing antibody-drug conjugates or fluorescently labeling proteins, using NHS eases the workflow. Minor tweaks in reaction conditions—choice of base, temperature, and solvent—tailor the selectivity and speed of modification, which drives efficiency in both bench and industrial settings.
Practitioners know N-Hydroxysuccinimide by more than its IUPAC name. Synonyms include NHS, 1-Hydroxypyrrolidine-2,5-dione, and Succinimide-N-oxyl. Commercial catalogues may list it by multiple identifiers, referencing CAS numbers, EINECS codes (228-199-0), or alternative nomenclature. In the field, calling it simply “NHS” is the norm, especially during fast-paced lab sessions or when negotiating bulk orders for largescale synthesis. Seeing the term "NHS ester" means the product is ready to transfer its moiety to another molecule, showing its modification potential beyond the plain compound.
Through years in the lab, safety with NHS never left the back of my mind. It presents as a moderate irritant—nothing dramatic, but enough to sting eyes and skin. Labels warn users to avoid dust, ensure good ventilation, and steer clear of ingestion. Routine use calls for nitrile gloves and goggles, plus a lab coat. Material Safety Data Sheets (MSDS) highlight risks: decomposition at high temperatures, potential for nitrogen oxides to form in a fire, and the need for good ventilation. Labs install spill kits nearby and keep wash stations accessible. Waste disposal requires segregating NHS-contaminated materials, usually sending them for chemical incineration, not dumping them in regular trash or drains. Years of lab audits push organizations to review handling guidelines every quarter, which keeps incidents rare.
Nowhere does NHS outperform expectations quite like in bioconjugation. Chemists depend on it to activate dyes, drugs, or linkers for attaching to proteins or peptides. It provides the backbone for many diagnostic tools—think about nearly every modern immunoassay or targeted therapy requiring site-selective labeling. Pharmaceutical companies deploy NHS for antibody-drug conjugate (ADC) production, maximizing payload delivery to specific cells. The material also finds its way into polymer modification for drug delivery, imaging agent production, and biosensor development. Each year I’ve witnessed new methods pop up, from cleavable linkers to photostable NHS derivatives, continually expanding its scope.
Universities and biotech firms dedicate significant effort exploring new reactions and more selective derivatives based on the NHS scaffold. The race to develop cleavable linkers in targeted therapies, for example, roots itself in modifying standard NHS esters to allow timed release. Enzyme-sensitive groups or photoactivatable modifications trace back to the original ring structure of NHS, showing just how versatile the core compound remains. In a world moving toward more personalized medicine, research teams keep searching for NHS analogs that provide better specificity or less off-target reactivity. I’ve seen papers each year investigating NHS for everything from creating safer imaging agents to new biocompatible implants, reflecting its trusted backbone and the innovative ways that chemists use it.
Toxicological assessment remains vital, even for seasoned researchers who use NHS routinely. Its acute toxicity sits in the moderate range, with LD50 values in rodents showing mild to moderate harmful effects with large doses. Chronic exposure risks remain less clear, driving regulatory agencies to request more data as NHS-based technologies enter clinical use. Community conversations in occupational safety settings highlight the importance of limiting dust formation and frequent glove changes. NHS breaks down in the environment to succinimide and other compounds, raising questions about long-term ecological impact, although no large environmental incidents have been connected to routine lab use. Still, labs set up procedures based on both direct toxicology data and good old-fashioned precaution, requiring secure waste collection and regular air monitoring.
Advancements in biotechnology and precision medicine keep NHS at the edge of chemical innovation. New NHS derivatives now offer enhanced reactivity, bioorthogonality, or improved solubility. Green chemistry advocates work on alternative preparation methods using less hazardous reagents or producing fewer byproducts, aiming to cut down both cost and environmental footprint. In the pharmaceutical pipeline, NHS esters remain crucial for the next generation of targeted ADCs, bioconjugates, and polymer–drug conjugates. Automation in process chemistry means larger batches produced with tighter control, less waste, and lower worker exposure. The next horizon—smart biomaterials or site-specific imaging probes—heavily relies on NHS-type chemistry, and it's likely that the tried-and-tested molecule will spearhead innovation just as it has for the last fifty years.
Scientists often need a way to link two molecules together. N-Hydroxysuccinimide, a white powder with a strange name, steps in to make that possible. You won’t see people talking about it on the news, and it rarely gets credit outside technical journals. But ask any biochemist or someone involved in making medical tests, and they’ll tell you how important it becomes once you’re in the lab.
In real life, medical breakthroughs don’t just depend on new discoveries—much of the magic happens in the chemistry that ties everything together. During protein research and antibody development, scientists use N-Hydroxysuccinimide mainly to activate carboxyl groups. This action allows molecules to bond, which forms the building blocks of test kits, targeted drugs, and diagnostic markers.
You’ll find it most in a reaction called NHS esterification. A researcher working with a protein, aiming to attach a fluorescent label for tracking, will add N-Hydroxysuccinimide to the process. The reaction it starts keeps the label stuck firmly to the protein, so the experiment doesn’t fall apart during testing. The science might sound complicated, but the goal is simple: see more, measure better, build more reliable results.
I spent some time working with a team developing detection tools for food safety. We used test strips to check for allergens in processed food. In each strip, a labeled antibody—stuck in the right spot only because of a reaction with N-Hydroxysuccinimide—searched for peanut proteins. Without N-Hydroxysuccinimide, those antibodies slid off or failed to grab the label, and test accuracy dropped fast. That’s one clear example of how dry chemical knowledge shapes public safety.
Labs working on drug delivery research also rely on this chemical. Designing targeted therapies for cancer, for example, means linking drugs to antibodies that seek out tumors. N-Hydroxysuccinimide helps glue everything together at the molecular level. Without that link, expensive cancer treatments risk hitting the wrong cells, causing more harm than good.
Working with N-Hydroxysuccinimide brings up questions. Lab safety stays high on the priority list because chemical exposure always comes with risks. Researchers follow strict protocols, and the industry pushes for greener alternatives. There’s a movement to reduce chemical waste and make processes safer. Some startups and university labs search for bio-based coupling agents, using what we learn from nature to improve the process.
Chemical supply chain disruptions have caused shortages of basic reagents in recent years, especially during global crises. Biomedical progress depends on making sure these unsung chemicals remain available. Diversifying sourcing and supporting more regional manufacturers would help labs continue their work without sudden gaps.
N-Hydroxysuccinimide doesn’t grab headlines or trend on social media. Still, for researchers, doctors, and patients who count on effective medical tests or new treatments, its behind-the-scenes role makes a real difference. Investing in better chemistry, stronger safety, and more secure supply chains will keep progress moving forward, one molecule at a time.
Some chemicals in the lab require extra respect. N-Hydroxysuccinimide, often known as NHS, falls into this group. You only need one bad batch for things to go sideways and to realize the money, time, and even safety you lose with casual storage. Researchers and lab managers who cut corners end up dealing with weakened reagents or unreliable experiments. From my years managing graduate-level research and routine quality control batches, it’s clear that storage decisions shape outcomes, and this seemingly small step ripples through the most ambitious projects.
NHS does not forgive exposure to moisture. This molecule draws in water from the air, and the resulting hydrolysis robs it of its chemical punch. Open the bottle too often or leave it unsealed, and soon you might spot clumping or discoloration—a telltale sign of degraded material. I learned early in my career that keeping NHS in tightly sealed containers saves more than just the expense of a replacement order. It preserves accurate, reproducible work.
Any lab with a reliable outcome stores NHS in cool, dry conditions, away from direct sunlight. Specialized desiccators work, but a simple airtight bottle paired with a desiccant packet does the trick in most cases. Once, during a hectic month on a synthesis project, someone left the reagent bench stock by the window. Even minor sunlight transformed a small jar’s contents, leaving us with waste, not a reagent. Direct sun exposure speeds up degradation. Room temp works for short-term use. For long-term needs, many labs stash it in the fridge or a dedicated cold storage unit, usually between 2–8°C. Deep-freezing isn’t required for NHS, yet lower temps slow down any decay. Locking it inside a fridge (not the freezer) along with a sturdy desiccant controls humidity without inviting frost or clumping.
NHS should carry clear labels with the open date and source. Any ambiguity about how long a bottle’s been in rotation can mess up recordkeeping, which in turn threatens project integrity. Routinely, I kept a simple logbook for regulated chemicals, cross-checking the lot number and tracking expiration to avoid using compromised stock. Powder should only be removed with dry, clean tools. Avoid scooping directly from the main bottle in humid rooms—single-use aliquots in tightly capped vials sidestep cross-contamination and keep the rest dry. Some teams keep small portions at the ready, restocking from the main supply only when needed.
Safety and research guidance reinforce storing NHS in sealed, moisture-proof containers, away from direct light and heat. According to documented chemical hazard sheets, accidental degradation releases byproducts that serve no use to research or industry and may pose health or waste disposal issues. In any lab I worked, responsibility didn’t end with good inventory—frequent checks for discoloration or clumping stopped accidents before they affected results.
Solid chemical storage habits reflect a culture of doing work you can trust. One time, a friend’s project on protein cross-linking came close to being derailed by NHS that was improperly stored by a previous shift—results became erratic, wasting precious days. Keeping chemicals dependable is as important as scientific skill. Simple habits—tight lids, cool shade, careful labeling—make the difference between a smooth workflow and a costly mess.
Ask anyone who’s worked in a life sciences lab about N-Hydroxysuccinimide, and you’ll likely get a nod of recognition. It goes by the short and simple term “NHS” more often than not. This small molecule has big relevance. Its structure forms the backbone of countless chemical reactions, connecting proteins, peptides, and all sorts of active components in research and medicine. But what really gives NHS its edge? The core lies in how the chemical groups fit together.
Take a moment to picture a five-membered ring—think of a pentagon. In N-Hydroxysuccinimide, that ring consists of four carbon atoms and one nitrogen. The proper term here is a “succinimide” ring. Two carbonyl groups hang off the ring, attached to the carbons at the 2 and 5 positions. The nitrogen up top doesn’t just stay quiet. It links directly to a hydroxy (–OH) group. This creates the N-hydroxy modification.
Chemists write the chemical formula as C4H5NO3. The unique combination – that ring structure with the extra hydroxy group – makes the molecule highly reactive, ready to leap into action in a water-based environment.
I remember my first try at coupling a protein with NHS. I’d run through the theory in class, but holding that white, crystalline powder made all the difference. In a simple buffer, NHS teams up with carbodiimide reagents (like EDC or DCC). The carbonyl groups on the ring draw in carboxyl groups like a magnet. Through this teamwork, NHS transforms itself into an active ester. This step allows for a new bond between proteins or other bioactive compounds. It’s the structure, not just the sequence of atoms, that enables all this.
The ring confers stability, but not so much that it clings too tightly. NHS esters hover right at the sweet spot: long enough to get where they need, but not so stable that the reaction stalls. Hospitals, research labs, and industrial chemists all lean on this property. NHS has shaped fields from targeted drug delivery to everyday diagnostic kits.
Safety data from the European Chemicals Agency shows NHS ranks among lower-risk chemicals if you’re careful. Handling still calls for gloves and goggles—dust can irritate, and nobody wants lab mishaps.
The molecule’s molecular mass sits at 115.09 g/mol, small enough for easy measurement and mixing. The melting point hovers around 95 °C, so it stays solid through routine lab work.
Some drawbacks crop up. The same reactivity that helps NHS in synthesis can lead to wasted reagents if moisture or heat sneaks into your stock. I’ve seen colleagues lose a batch to an unsealed bottle and humid weather.
Solutions rest in good practice: airtight containers, silica packs, quick hands when weighing samples. For those outside academia, robust packaging and shelf-life labels make a real difference. Education—showing new researchers why the structure matters—remains critical. NHS isn’t just a tool, it’s a lesson in how small tweaks in structure unlock major breakthroughs.
N-Hydroxysuccinimide, often called NHS in labs, plays a big role in fields like biochemistry and pharmaceuticals. It gets used to help chemical reactions go faster, especially when people need proteins and other molecules to link together. You probably won’t see NHS outside lab settings. Scientists love it because it simplifies many experiments, making their results more reliable.
Most people never run into N-Hydroxysuccinimide at home or in their daily jobs. In labs, though, exposure can happen through skin contact, breathing dust, or—even less often—accidental swallowing. NHS appears as a white, crystalline powder, and tiny particles can float around if it’s not handled carefully. I remember my own days in the lab when one careless spill sent colleagues scrambling for gloves and goggles. People treat this chemical with respect, knowing direct exposure shouldn’t be taken lightly.
Research on NHS in people stays limited. Animal studies and experience in labs suggest it can irritate skin, eyes, and lungs. Breathing in even a small amount can lead to coughing or shortness of breath, and skin contact might cause a rash or redness. Eye exposure? That’s always trouble, with burning or watering. Swallowing NHS brings more severe symptoms—nausea, stomach pain, and discomfort—though reports stay rare since most people handling it use proper safety gear.
The European Chemicals Agency classifies NHS as hazardous, mainly for its potential to irritate, but it's not considered a cancer risk or a poison in small amounts. Still, the margin for carelessness is slim. In real-world settings, accidents usually happen when safety routines slip—a glove with a small tear or a fume hood not turned on. I have seen people learn the hard way once and never repeat the blunder.
Chemical safety boils down to straightforward habits. Gloves, goggles, and lab coats keep NHS on the outside. Good ventilation or fume hoods keep dust out of the air. Labels and clear instructions help everyone know what they’re dealing with. The right habits don’t just keep you out of harm’s way—they foster a safer, more mindful work culture. From my own experience, mistakes teach you fast, but working with teams that value safety saves a lot of worry and pain.
Addressing health risks means focusing on education. Everyone handling NHS should get hands-on safety training, not just a set of guidelines. Supervisors need to inspect safety gear often and replace damaged equipment right away. Clear documentation and well-marked containers keep people from confusing NHS with less hazardous chemicals. Companies can put routine health checkups on the calendar for staff who handle chemicals every day.
If you work with NHS, washing up before leaving the lab, never eating or drinking nearby, and reporting any spills on the spot help keep minor incidents from turning into bigger problems. The key isn’t to fear N-Hydroxysuccinimide but to treat it with the seriousness it deserves.
To sum up, NHS does come with health risks, mostly around irritation from dust or skin contact. Scientists have learned safe practices through years of handling. Good habits, proper training, and the right equipment keep problems rare. People outside labs hardly ever face risks from NHS, but for researchers and lab techs, treating this chemical with respect goes a long way.
N-Hydroxysuccinimide, better known as NHS, sits on the shelves of nearly every biochemistry lab with good reason. People tend to reach for NHS when they want to bring two molecules together in a controlled, predictable fashion. Bluntly put, it acts like a skilled matchmaker for proteins and other biomolecules. The main action comes from forming stable amide bonds, connecting amines—typically a lysine side chain on a protein—to carboxylic acids found on various substrates. In the real world of test tubes and microplates, this often means linking an antibody to a fluorescent dye or an enzyme to a solid surface for later detection work.
Take an ELISA kit or a rapid test, and odds are high you’ll find something linked together with NHS chemistry. The NHS ester reacts briskly with exposed primary amine groups on proteins. Researchers choose this route not just for its reliability, but also because it creates a strong enough connection to stand up to repeated washes and stringent detection protocols. People designing assays rely on this stability to secure their capture antibodies to microplate wells or magnetic beads, knowing the assay won’t fall apart during use. NHS makes sure test results hold up under pressure, giving clear answers to tough biological questions.
The story doesn’t stop at diagnostics. Drug developers run into the challenge of getting potent molecules exactly where they’re needed, and NHS-enabled conjugation gives them a way to strap targeting ligands to therapeutic agents. This makes for smarter drug delivery, tailoring treatments to go after cancer cells, for example, without scattering toxicity throughout the body. Researchers have used NHS esters to connect monoclonal antibodies and peptides to small-molecule drugs, building antibody-drug conjugates that bring cancer treatments directly to tumor cells. Precision in coupling here means fewer side effects and more powerful shots at the real target.
Many protein purification and detection strategies revolve around the powerful biotin-streptavidin interaction. Attaching biotin to a protein using NHS-biotin reagents turns a regular protein into one that sticks tightly to coated beads or plates. This switch transforms basic proteins into sharp tools for affinity purification, pull-down assays, and various biosensor systems. In the hands of skilled experimenters, what started as plain NHS becomes a workhorse for separating, visualizing, and manipulating proteins in complex mixtures.
Getting a real look at how protein complexes hold together has grown more precise thanks to NHS-based crosslinkers. Short, reactive linkers featuring NHS groups make it possible to lock interacting proteins in place, letting structural biologists freeze-frame a protein’s handshake with its partner. Crosslinking then leads right into mass spectrometry or crystallography, where researchers sort out the detailed shapes of these molecular assemblies. The hope is that with better maps, people can design new interventions for diseases driven by protein misfolding or unwanted protein interactions.
Like any strong chemical tool, NHS asks for a cautious hand. Overexposure or improper disposal risks harm, so safety measures in the lab become non-negotiable. Researchers use NHS for its specificity and utility but also respect the need for proper ventilation, gloves, and timely waste management. Honoring the health of everyone in the lab goes hand in hand with scientific progress.
From diagnostics to therapy development and protein research, NHS plays an outsized role in shaping what’s possible in biochemistry. Speaking from time at the bench, reliable coupling really does make the difference between chasing possibilities and delivering on results. Whether building assays that diagnose infections early or inventing new lines of attack against cancer, a pinch of NHS often marks the first real step from idea to working solution.
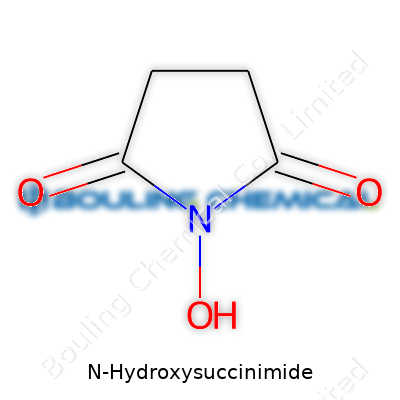
| Names | |
| Preferred IUPAC name | 1-Hydroxy-2,5-pyrrolidinedione |
| Other names |
NHS 1-Hydroxy-2,5-pyrrolidinedione Succinimide-N-oxyl N-Hydroxysuccinimide, 98% Hydroxysuccinimide |
| Pronunciation | /ɛn haɪˌdrɒksi səˈkɪnɪmaɪd/ |
| Identifiers | |
| CAS Number | 6066-82-6 |
| Beilstein Reference | 1206490 |
| ChEBI | CHEBI:45678 |
| ChEMBL | CHEMBL1419 |
| ChemSpider | 579 |
| DrugBank | DB08797 |
| ECHA InfoCard | N-Hydroxysuccinimide ECHA InfoCard: 100.003.406 |
| EC Number | 3.2.2.23 |
| Gmelin Reference | 71552 |
| KEGG | C02335 |
| MeSH | D017929 |
| PubChem CID | 822 |
| RTECS number | WH6940000 |
| UNII | R1J1J59T1O |
| UN number | 2811 |
| CompTox Dashboard (EPA) | DTXSID2020687 |
| Properties | |
| Chemical formula | C4H5NO3 |
| Molar mass | 115.09 g/mol |
| Appearance | White to off-white crystalline powder |
| Odor | Odorless |
| Density | 1.405 g/cm³ |
| Solubility in water | soluble |
| log P | -0.99 |
| Vapor pressure | 0.0065 mmHg (25°C) |
| Acidity (pKa) | 6.0 |
| Basicity (pKb) | 6.1 |
| Magnetic susceptibility (χ) | -49×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.471 |
| Dipole moment | 4.36 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 200.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | –482.0 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -1571 kJ/mol |
| Pharmacology | |
| ATC code | N01AX |
| Hazards | |
| Main hazards | Harmful if swallowed, causes serious eye irritation |
| GHS labelling | GHS07, GHS06 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | Wash hands thoroughly after handling. IF ON SKIN: Wash with plenty of water. If skin irritation occurs: Get medical advice/attention. Take off contaminated clothing and wash it before reuse. |
| NFPA 704 (fire diamond) | Health: 2, Flammability: 1, Instability: 1, Special: - |
| Flash point | 86°C (187°F) |
| Autoignition temperature | 430 °C (806 °F; 703 K) |
| Lethal dose or concentration | LD50 (oral, rat): 5000 mg/kg |
| LD50 (median dose) | LD50 (oral, rat) = 5000 mg/kg |
| NIOSH | Not listed |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 mg/m³ |
| IDLH (Immediate danger) | Not listed |
| Related compounds | |
| Related compounds |
Succinimide N-hydroxysulfosuccinimide N-hydroxyphthalimide N-hydroxyimide 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide |