N-Ethylpyrrole came into the spotlight as researchers chased after new building blocks for drug discovery and specialty materials. Its parent, pyrrole, appears in a surprising number of old laboratory notes, but the ethylated version stepped forward in the twentieth century. Chemists, always on the hunt for compounds that push the boundaries of reactivity, began modifying the pyrrole core. It’s hard to ignore how quickly labs in both academia and industry turned to N-Ethylpyrrole once it became clear that the N-alkyl group could tweak reactivity, volatility, and solubility compared to standard pyrrole. By the mid-1900s, its role had expanded, especially as synthetic organic chemistry pushed forward the possibilities in pharmaceuticals and dyes.
N-Ethylpyrrole stands out as a five-membered heterocycle featuring an ethyl group tacked onto its nitrogen atom. Its liquid form, faint amine-like odor, and distinctively low viscosity make it an easy candidate for handling in bench-scale synthesis. It shows up in bottles labeled for research use, always carrying a warning label and a promise of synthetic flexibility. In chemical catalogs, it often appears with hints of its uses in advanced organic synthesis, specialty polymers, and even as a key intermediate for lab work on more complex heterocycles.
Spotting a sample of N-Ethylpyrrole, most chemists recognize its pale yellow hue and quick-to-vaporize nature. It boils near 132°C and carries a melting point so low it’s tough to freeze under everyday lab conditions. Its density matches most light organic liquids, swimming around 0.98 g/cm3. Flammability poses a risk: its flash point sits worryingly low, so a careless flickering flame in a poorly ventilated space becomes a problem. Solvent-wise, it blends with ethanol, ether, and other organics, avoiding water’s reach thanks to its nonpolar tendencies. As for chemical behavior, the ethyl group blocks some routes for hydrogen bonding but opens new doors in acylation and electrophilic substitution.
Lab labels for N-Ethylpyrrole often include CAS number 3973-19-5, highlighting purity ranges above 97%. Color can shift slightly depending on trace impurities, but reputable suppliers keep it clear of colored tars and peroxides. The safety sections frequently warn about both volatility and skin contact. I’ve seen labs store it under nitrogen or argon just to avoid slow oxidation, and manufacturers bundle it in amber glass to take the edge off light-induced decomposition.
Swinging back to traditional preparation, most bench chemists reach for a base-promoted N-alkylation route. Begin with pyrrole, chill it down, then add ethyl bromide—carefully—while stirring with a non-nucleophilic base like sodium hydride. That exothermic fizz marks progress, though a rush can lead to over-alkylation and byproducts. Some prefer milder approaches, swapping the halide for ethyl sulfate, which tones down the violence but stretches out the reaction time. After solvent extraction and careful distillation, a clean fraction of N-Ethylpyrrole ends up in the receiving flask, ready for further transformation.
Looking at modifications, N-Ethylpyrrole sits at a crossroads of reactivity. The ethyl group shields the nitrogen, encouraging chemists to go after the carbon atoms of the ring for further substitution. Pour in strong acids and you risk polymerization, but controlled electrophilic aromatic substitution leads to halogenation, nitration, or acylation, depending on the reagents and conditions. Metal-catalyzed cross-couplings let you stitch on aryl or alkyl side chains. The electron-rich nature of the ring means oxidative coupling can lead straight into polypyrrole derivatives, prized in conductive polymers.
On a chemical shelf, you’ll spot N-Ethylpyrrole hiding under a handful of aliases: 1-Ethylpyrrole, Pyrrole, N-ethyl-, or 3973-19-5, and sometimes even as a key ingredient in specialty chemical blends. Some catalogs drop in foreign language versions, especially in European and Asian markets. Regardless of the name, every bottle tells the same story: a niche compound, but a flexible one.
Reliance on N-Ethylpyrrole demands a clear-eyed view of its hazards. Skin exposure brings mild irritation, and its vapors pose respiratory risks, particularly for those used to working glove-free or skimping on ventilation. I’ve always kept fume hoods going out of habit, and spills demand quick action thanks to the flammable vapor. Storage demands amber glass away from oxidizers and ignition sources. Comprehensive safety data sheets insist on splash goggles, gloves, and proper waste containers, making clear that safety in handling separates careful researchers from the rest. In larger facilities, regular monitoring for vapor leaks and strict tracking of chemical inventory reduce both risk and loss.
N-Ethylpyrrole finds a place in the toolkit of medicinal chemists and materials scientists. On the pharma side, it forms the backbone of drug candidates looking for central nervous system activity or anti-inflammatory kick. It’s a helpful intermediate for tweaking the electronic properties of molecules in search of the right fit for enzyme binding pockets. In materials, researchers combine it with other monomers to spin out polypyrrole derivatives for batteries, antistatic coatings, and sensors. Some ink manufacturers have experimented with N-Ethylpyrrole-based dyes to sharpen colorfastness and stability. The expanding reach of organic electronics keeps it in circulation as new conductive films and organic LEDs emerge from the lab.
Recent years have seen a steady stream of research probing both fundamental and applied chemistry of N-Ethylpyrrole. Groups worldwide focus on new routes for catalytic functionalization, aiming to cut down both cost and waste. Publications on ring-substituted ethylpyrroles report on improved properties for organic transistors, while toxicologists push for greener modifications that soften environmental impact. I’ve attended more than one conference where presenters highlighted N-Ethylpyrrole-based scaffolds showing antiviral activity, fueling early-phase trials for infectious disease drugs. Each year, research dollars flow toward better understanding, scaling, and application of this versatile compound.
Toxicologists keep a close eye on N-Ethylpyrrole’s impact since many pyrrole derivatives slip past basic safety screens. Animal studies suggest mild acute toxicity with high-dose inhalation, but chronic data remain patchy. Work in environmental fate points out bioaccumulation stays low, though its breakdown products still warrant scrutiny. Increased interest in green chemistry sometimes leads to safer alternatives, especially for worker-heavy settings. Handling risks come mostly from its volatility, flammability, and the chance of accidental ingestion. I’ve watched colleagues double-check gloves and push for closed systems after small spills or splash incidents, a testament to both good habits and a respect for the unknowns still lurking in the toxicological profile.
The outlook for N-Ethylpyrrole keeps looking brighter as specialized chemistry carves out more space for creative molecular design. As the demand for custom electronics, high-density batteries, and environmentally safer pharmaceuticals grows, this molecule remains squarely in the frame. New synthetic upgrades—achieved through cleaner, low-waste processes—promise broader commercial reach and lower cost. Rising pressure to reduce toxic byproducts shapes both how chemists prepare it and how regulators view its use in large-scale manufacturing. Younger researchers coming into the field seem increasingly keen to unlock fresh applications for N-Ethylpyrrole, especially in molecular imaging, optoelectronics, and as part of smart biomaterials platforms. Between evolving regulation, better synthesis, and an eye for sustainability, the future of N-Ethylpyrrole continues to draw equal parts caution and excitement.
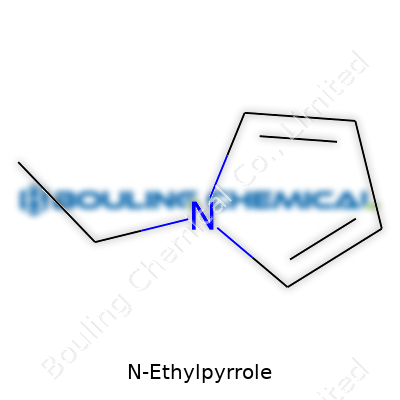
Some chemicals come with names that sound like a riddle only scientists want to solve. N-Ethylpyrrole falls into that camp, at first glance. But a closer look clears things up. The backbone is a five-sided ring of four carbons and one nitrogen—call it a pyrrole ring. That’s the same skeleton you’ll find at the heart of hemoglobin in your blood. Toss an ethyl group—two carbons and a handful of hydrogens—onto that nitrogen, and it becomes N-Ethylpyrrole.
The molecular formula tells the real story: C6H9N. Picture it: six carbon atoms, nine hydrogens, one nitrogen atom, all packed neatly into a compact shape. In terms of structure, draw the pyrrole ring and swap the plain hydrogen that usually hangs off the nitrogen for an ethyl group. The result? A tweak that totally changes the game compared to basic pyrrole.
For anyone who’s spent time around a bench or smelt solvents in a chemistry lab, small changes in a molecule often mean fresh properties—or stronger smells. N-Ethylpyrrole isn’t just a curiosity; chemists don’t just play with ring systems for fun. By adding an ethyl group, the pyrrole ring suddenly acts differently in reactions. You get a bump in solubility in certain organic solvents, and sometimes a shift in where it prefers to bond or react.
From what I’ve seen making heterocycles in lab work, tweaks like this can boost stability under some conditions, make molecules more or less eager to link up with others, or even change their potential as starting points for making new drugs. Life-saving medicines, dyes, and even flavors often begin with a few basic tweaks to old, familiar skeletons like pyrroles.
Sifting through the chemical catalogs for N-Ethylpyrrole, you won’t see it rivaling ethanol or benzene in terms of popularity. It doesn’t get the fanfare, even though simple pyrrole rings are everywhere in natural compounds. The main reason is that chemists still find it easier to start from simpler rings and tailor them as they go, rather than stockpiling every possible version.
One challenge: it takes careful technique to attach an ethyl group right where you want it. This often calls for controlled conditions, quality reagents, and a good hood fan (nobody enjoys the smell of raw pyrroles). In my own experience, sticking alkyl groups onto a ring so that it's the nitrogen that takes it (instead of a carbon) takes both steady hands and a lot of patience with yields that don’t always wow you.
Plenty of chemical industries benefit by making derivatives like N-Ethylpyrrole easier to access. There’s a strong case for refining old routes or finding greener ways to tack on alkyl groups. Reducing waste, cutting down on noxious byproducts, and improving yields sat high on our list each year in lab meetings.
Bringing in automation, combinatorial chemistry, and new catalysts can help. Chemists are always trying to move away from using harsh conditions or reagents that leave a mess behind. Cleaner, faster, repeatable chemistry isn’t just a dream—labs have made leaps in the last decade with more efficient N-alkylation reactions.
N-Ethylpyrrole isn’t going to sweep across news headlines, but it quietly underpins more chemistry than many suspect. A simple tweak gives this ring new life and utility, whether as a building block or a tool in the search for new molecules that work better, faster, or safer. In the end, structure matters at every level, from the lab bench to real-world applications.
Chemical names usually spark people to tune out. N-Ethylpyrrole doesn’t exactly roll off the tongue, either. Still, this small organic compound quietly fuels parts of our daily lives. You wouldn’t find it on the supermarket shelf, yet plenty of folks benefit from it without knowing.
Here’s the neat part: N-Ethylpyrrole finds a spot in flavor and fragrance chemistry. Most artificial flavors and scents build off a web of small molecules. N-Ethylpyrrole brings a roasted, nutty, and somewhat earthy note, so product developers reach for it to round out coffee, cocoa, and meaty flavors. Some food chemists describe it as a hidden player in the illusion of ‘real roast’—think kettle-roasted peanuts, hearty chocolate, and even the scents wafting from barbecued foods.
Manufacturers dabble in tiny concentrations to get those notes just right. The amounts floating into your coffee aroma hardly register as a health risk according to existing toxicology reviews, but the difference it makes to the flavor profile can’t be ignored. A lot of people who enjoy processed snacks, flavored chips, or rich sauces have encountered its handiwork, even if the packaging skips the chemistry details.
Away from your kitchen, N-Ethylpyrrole’s chemical backbone supports paint and ink tech. Formulators add small, specialized molecules to control drying times, color stability, and how inks adhere to surfaces. I’ve seen technicians experiment with pyrrole derivatives to tweak how an ink prints on different packaging films. Adjusting the base chemicals helps companies get crisper images on everything from soda cans to flexible food wraps. N-Ethylpyrrole isn’t always the star, but it often helps make these blends work without smudges or faded colors.
Electronics manufacturers count on certain pyrroles for assembling tiny, conductive polymers. These new plastics end up in flexible displays, touch screens, and solar panels. Polymers built with the help of N-Ethylpyrrole can push electrical signals better than old-school plastics. As devices get smaller and more flexible, this kind of material science solves old engineering headaches, opening up products like rollable tablets and wearable sensors.
Medicinal chemistry leans heavily on building blocks like N-Ethylpyrrole. Drug discovery always starts messy, with exotic core molecules slotted in to test out biological effects. Scientists shuffle side groups around to see how minor changes affect activity, and N-Ethylpyrrole surfaces as a handy starting point. Certain antifungal, anti-inflammatory, and antimicrobial leads feature structures based on pyrroles, including the ethylated form. Even if the medicine reaching your pharmacy never lists this compound, steps in its synthesis might have depended on it earlier.
No chemical is perfect. While N-Ethylpyrrole isn’t flagged as a major toxin, the usual safety rules apply around industrial settings: good ventilation, careful storage, and personal protective equipment. On the environmental side, responsible disposal and traceability should stay top-of-mind. Some countries want tighter rules for flavor chemicals and ink additives, making transparency and oversight more crucial as new uses keep appearing. Industry groups push for better testing so we’re not guessing at long-term effects. Users and makers benefit when the details stay public and research keeps moving forward—whether the compound ends up on a flavor wheel or in a roll of flexible electronics.
N-Ethylpyrrole isn't a household name, but if you spend time in a lab or chemical plant, the stuff can find its way onto your shelves. I spent a few years in an industrial lab—if there's something I learned, it's that even small bottles demand respect. N-Ethylpyrrole carries a reputation for volatility, and overlooking proper storage can turn a smooth day into a scramble for the spill kit.
Most experienced chemists don't leave anything to chance. This chemical reacts with oxygen pretty quickly, so it’s not wise to leave it out in the open. Sealed containers make sense here—not just capped, but checked for leaks or damage before every use. Flammable cabinet storage helps keep it away from heat; it doesn’t take much to ignite vapors, especially in a crowded workspace. If you've ever watched a co-worker fumble with a corroded, sticky cap, you know how fast something routine can become risky.
My early days working with specialty chemicals taught me how a small slip-up can snowball. Just last year, a new hire ignored gloves thinking, “It’s just a quick transfer.” She ended up with a nasty skin irritation. N-Ethylpyrrole absorbs through the skin, so gloves are non-negotiable. Splash goggles and lab coats aren’t overkill; they're insurance. Fume hoods get all the praise for organic solvents, but here, the need is real—breathing in the vapors can irritate the lungs and eyes.
You can’t rely on instincts alone. MSDS sheets haven’t gone out of style, and for good reason. Double-checking storage temperature matters. High ambient temps in summer, especially around older buildings without good climate control, can raise the risk of evaporation and, with it, flammable vapors. Keeping a log of who’s accessed or moved the container is something more labs should enforce—accountability stops a lot of corner-cutting before it starts.
Mistakes around N-Ethylpyrrole cost more than embarrassment. I’ve seen minor spills turn an entire section of a lab into a no-go zone for the afternoon. The cleanup isn’t fun—open windows, fans running, and everyone hoping the offender learned their lesson. Some folks ignore the risk to shared air until it hits home. If a fume hood’s not pulling right or you skip a glove change, the exposure creeps up.
The right storage reduces headaches. Polyethylene containers often stand up to the job, but check compatibility charts; glass sometimes works, but only with the right seal. Store away from acids, bases, and oxidizers. If your shelf is cluttered or labels faded, that’s a red flag. I’ve made it a habit to walk through storage areas once a week, just to check—five extra minutes beats hours of incident paperwork.
I always push for teamwork in training. A chemical safety session every few months, run by someone who’s seen a spill or two, keeps everyone sharp. Posting clear storage guidelines with diagrams near the shelves helps—pictures cut down on language barriers and assumptions. Disposal plans matter as much as storage. Old N-Ethylpyrrole doesn’t belong down the drain or in regular trash, and hazardous waste pickups aren’t just an option—they’re a requirement.
Storing and handling N-Ethylpyrrole calls for real attention—no shortcuts, no rushing, and no overconfidence. If you treat the stuff casually, trouble finds you fast. Respect the rules, keep gear in reach, and look out for your coworkers. Chemical safety isn’t just paperwork—it’s everyone getting home with all ten fingers and no stories for the wrong reasons.
N-Ethylpyrrole doesn’t pop up in most people’s daily conversations, but it turns up in chemistry labs and specialty manufacturing. The compound looks unassuming: a colorless to pale yellow liquid, faintly reminiscent of the peculiar smell you get when working with solvents. Most folks won’t see it outside a controlled environment, but that doesn’t mean it comes risk-free.
Health agencies don’t recognize N-Ethylpyrrole as a mainstream hazard the way they do with heavy metals or known carcinogens. No splashy warnings at the consumer level, no panic in workplace safety bulletins. Even so, absence of horror stories isn’t a green light to take it lightly. The science tells us that pyrroles as a group can mess with the liver and nervous system given enough exposure. N-Ethylpyrrole hasn’t been studied as deeply as some cousins like pyrrole itself, but chemical cousins often share more than just a name.
A safety data sheet typically labels N-Ethylpyrrole as irritating to skin, eyes, and respiratory tract. These aren't exaggerated warnings. Even mild skin contact can set off a rash or leave your hands tingling. Breathing in the vapors can sting your eyes and throat. In my own university days, I watched a classmate cough up a storm during an experiment where the hood ventilation failed to catch the fumes. She shook it off, but nobody forgot the lesson.
Spend some time searching through academic databases on N-Ethylpyrrole, and you’ll find the information is thinner than it should be for public comfort. So far, major health agencies haven’t listed long-term cancer risks, but it’s tough to rule out trouble before more research comes in. That lack of information weighs on people working with it, because history is full of chemicals that looked “safe” until years later.
One thing we know: just a few milliliters on your skin or a quick inhale of fumes can leave anyone feeling queasy. Over repeated exposure, you might see respiratory irritation get worse, maybe even signs of organ toxicity if you’re unlucky and careless about personal protection. For those mixing or storing containers in manufacturing plants, simple mistakes — missing gloves, open bottles — bring quick reminders that chemical safety isn’t optional.
It takes more than reading labels to work confidently with any chemical. People tend to skip the goggles or forget about the mask when pressure mounts in the lab, but N-Ethylpyrrole serves as a clear vote for caution. You won’t find bulletproof data pointing to cancer or birth defects, but no news isn’t the same as good news. Proven steps like using gloves, eye protection, and working in a space with solid ventilation make sense until the science fills in the blanks.
Companies and labs could do better by treating N-Ethylpyrrole with extra respect rather than saving safety measures for dramatic substances. Minute traces of vapor will always slip out. Better training, regular reviews of current research, and honest communication make a real difference. Even when a chemical doesn’t top the danger charts, nobody deserves to face mystery symptoms from a poorly labeled bottle or a shortcut taken under deadline.
At a policy level, waiting for concrete proof of severe harm can leave workers and researchers wide open to health surprises. The smart approach follows the “better safe than sorry” principle. That means updating material safety guides as soon as credible data surfaces, swapping in less risky substitute compounds when possible, and nudging suppliers and regulators to keep digging for evidence.
In my experience, respect for chemicals like N-Ethylpyrrole doesn’t lead to overkill. It’s about taking everyday steps that stack up to real protection, long before anyone lands in the ER. The world’s collection of industrial chemicals grows every year, but the common practice of basic, consistent safety works for all of them — including the ones still flying under the radar.
Anyone dealing with fine chemicals knows how much purity matters. Even a small impurity in a compound like N-Ethylpyrrole can change reaction outcomes, upset a sensitive experiment, or skew the results scientists rely on. Researchers and manufacturers look for purity levels of at least 98% for N-Ethylpyrrole. Sometimes, suppliers stretch up to 99% if a process really requires it. These numbers don’t get tossed around lightly. I once worked through a project that depended on just one percent purer material, and the difference that made was the difference between a publication and having to start over.
Testing for purity usually runs through gas chromatography or NMR, since a bit of leftover starting material, an odd solvent residue, or moisture can cause a headache down the line. Labs expect purity listed right on the bottle — not only for peace of mind, but so they know what to account for. A clean label reading “98%” has a ripple effect through someone’s workflow, and there’s little patience out there for mystery grades.
People rarely need N-Ethylpyrrole by the drum. It often lands in labs or pilot plants where demand isn’t enormous, so most suppliers ship it out in small bottles. The most common bottle sizes are 1 gram, 5 grams, 10 grams, and 25 grams. On rare occasions, you’ll even see a 100-gram bottle available, but anything above that seems reserved for those who know exactly what they are doing with bulk orders.
Among chemists, this sizing pattern isn’t just habit — it keeps the material fresh. Once the cap pops off, air and moisture become the enemy. N-Ethylpyrrole carries enough volatility and reactivity to justify careful handling, and repackaging into small containers is actually more of a safety move than a sales gimmick. One 10-gram bottle goes a long way in synthesis work or reference testing, and I’ve seen whole projects operate off a box of 5-gram batches.
There’s also shipping to think about. Chemicals like this must meet transport rules; they often get shipped in glass amber vials, sealed tightly, sometimes in metal cans for added protection. In a pinch, a supplier may split a bulk amount into multiple small vials so none of it goes to waste — and if you’ve ever spilled a pricey reagent, you know why this matters.
For N-Ethylpyrrole, purity supports not just performance but also health and safety. Lower grades could carry unknown byproducts, complicating analysis and making workplace cleanliness harder. Most buyers are looking for a minimum of 98% — a simple assurance that the product will act the way it’s supposed to. Sometimes a supplier will specify “analytical grade” or “reagent grade,” but in the end, those numbers on the specification sheet say more than a marketing phrase ever could.
On the packaging side, lab managers are trying to keep shelf space under control, finances in check, and waste minimized. Smaller vials suit the stop-start rhythm of research and prototyping. Even a little extra care in packaging can help cut down waste and lower risks for those working hands-on with reactive chemicals.
A few things help buyers get what they need without getting burned. Suppliers that post solvency and impurity specs, offer photos of actual containers, and provide batch certificates tend to be the ones repeat customers come back to. Clarity on purity and exact container size lets labs keep things tight, costs low, and mistakes rare. So if you ever order N-Ethylpyrrole, look past the catalog title and see what the supplier writes in small print — it saves headaches in the future.
| Names | |
| Preferred IUPAC name | 1-ethyl-1H-pyrrole |
| Other names |
1-Ethylpyrrole N-Ethyl-1H-pyrrole |
| Pronunciation | /ɛn-ˈɪθ.ɪl.pɪˌrɒl/ |
| Identifiers | |
| CAS Number | 617-89-0 |
| Beilstein Reference | 1430848 |
| ChEBI | CHEBI:52006 |
| ChEMBL | CHEMBL41638 |
| ChemSpider | 76666 |
| DrugBank | DB04268 |
| ECHA InfoCard | 100.013.746 |
| EC Number | EC 209-763-5 |
| Gmelin Reference | 9235 |
| KEGG | C08388 |
| MeSH | D053601 |
| PubChem CID | 12080 |
| RTECS number | UK4375000 |
| UNII | SVK6S0B848 |
| UN number | UN2524 |
| Properties | |
| Chemical formula | C6H9N |
| Molar mass | 109.16 g/mol |
| Appearance | Colorless to yellow liquid |
| Odor | amine-like |
| Density | 0.944 g/mL at 25 °C |
| Solubility in water | Slightly soluble |
| log P | 1.67 |
| Vapor pressure | 0.385 mmHg (25 °C) |
| Acidity (pKa) | 17.0 |
| Basicity (pKb) | 3.80 |
| Magnetic susceptibility (χ) | -49.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.505 |
| Viscosity | 3.62 mPa·s (20°C) |
| Dipole moment | 1.94 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 340.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -29.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2898.7 kJ/mol |
| Pharmacology | |
| ATC code | N05CM21 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Precautionary statements | P210, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-2-0-N |
| Flash point | 63 °C |
| Autoignition temperature | 315 °C |
| Explosive limits | 1.8–9.6% |
| Lethal dose or concentration | LD₅₀ (oral, rat): 373 mg/kg |
| LD50 (median dose) | LD50 (median dose): 960 mg/kg (rat, oral) |
| NIOSH | Not Listed |
| PEL (Permissible) | Not established |
| REL (Recommended) | 100 ppm |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
Pyrrole N-Methylpyrrole N-Phenylpyrrole 2-Ethylpyrrole Pyrrolidine |