The story of N-(Cyclopent-1-Ene-1-Yl)pyrrolidine traces its roots to a period marked by rapid advances in organic synthesis and a growing curiosity around nitrogen-containing heterocycles. Researchers, especially throughout the latter part of the 20th century, took a close look at pyrrolidine derivatives, searching for new frameworks that might push boundaries in medicinal chemistry. With the expansion of transition-metal-catalyzed amination and cyclization strategies, practical ways to access cyclopentene-fused rings and linkers emerged. Academic journals through the 80s and 90s point to a steady trickle of patents and papers, each adding bits of knowledge to the broader picture — often sparked by the promise of ring-fused systems for bioactive molecules. From a chemist’s bench, the motivation wasn’t always driven by industry. Sometimes a simple curiosity about how a five-membered ring on a pyrrolidine backbone might twist or react in different conditions got things going, often leading to an unexpected find in the process.
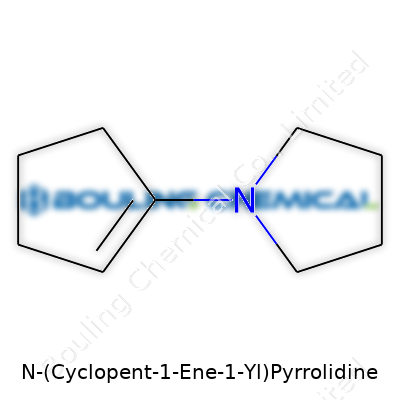
N-(Cyclopent-1-Ene-1-Yl)pyrrolidine represents a hybrid between two versatile five-membered rings: the unsaturated cyclopentene and the nitrogen-rich pyrrolidine. Structurally, attaching a cyclopentene moiety through the nitrogen atom lends this compound a unique blend of rigidity from the double bond and flexibility from the saturated pyrrolidine ring. Chemical suppliers catalog it as a specialty intermediate that offers both reactivity and a defined handle for downstream modifications. Scientists appreciate this molecule for more than its looks; its nitrogen provides basicity and the opportunity for hydrogen bonding, while the cyclopentene double bond acts as a reactive center. Across research benches, this product becomes a building block — one that expands the range of accessible analogs or scaffolds for drug or material discovery.
N-(Cyclopent-1-Ene-1-Yl)pyrrolidine usually arrives as a slightly viscous oil at room temperature, showing shades ranging from pale yellow to near colorless, depending on purity. Its boiling point runs higher than most simple amines, given the conjugated double bond and the five-membered rings’ core. In my time at the bench, I have noticed its sharp amine odor — comparable to other small-ring heterocycles but less pungent than piperidine. Its solubility profile looks friendly for most organic solvents; dichloromethane, ethyl acetate, and even methanol dissolve it quite well. In terms of stability, the nitrogen remains basic, and the double bond does open the door for reactions like additions or oxidations if not handled carefully. The compound won’t stand up to strong acids or oxidants — that double bond is a weak spot — so long-term storage works best in cool, dry, and inert conditions.
Labeling in the lab and in industry follows IUPAC conventions, but on most bottles, you’ll spot "N-(Cyclopent-1-en-1-yl)pyrrolidine", alongside the CAS number and formula. Commercial sources report purity by GC or NMR — 97% and above for most synthetic uses. Liquid density and refractive index data help with identification and quality control. Handling instructions focus on flammability and volatile amine vapors, along with suggestions for storage under inert atmospheres to curb peroxide formation or moisture pickup. Labels from trusted vendors lay out not only the identity but also the date of manufacture, batch number, and storage recommendations, so researchers know exactly what they’re working with.
On the synthesis front, the route calls for a blend of careful planning and practical know-how. One method starts with cyclopentenyl chloride, letting it react with pyrrolidine under basic conditions, often with a mild base such as potassium carbonate and a polar aprotic solvent like DMF or acetonitrile. Stirring the mixture, often overnight and at moderate temperature, brings reaction to completion. Extraction follows, then purification by distillation or column chromatography. I have seen some labs bypass the halide pathway, opting for reductive amination with cyclopentenone and pyrrolidine plus sodium borohydride or catalytic hydrogenation. Yields can swing from 60% to 85%, depending on scale and the tightness of procedural controls. Synthetic chemists sometimes add a pinch of sodium sulfate at the end, drying the organic layer before filtration and solvent removal.
N-(Cyclopent-1-Ene-1-Yl)pyrrolidine opens up intriguing chemistry thanks to its double bond and secondary amine. Hydrogenation over palladium wipes out the unsaturation, offering a saturated analog with distinct electronic properties. Selective oxidation targets the alkene or nitrogen, yielding N-oxides or epoxides—useful intermediates themselves. Alkylation and acylation on the nitrogen further tune the compound for specialty applications. The most creative folks I’ve worked with see this molecule as a way to introduce strain or ring tension, paving the way for cycloaddition or rearrangement reactions in total synthesis. The double bond also survives Michael-type additions with softer nucleophiles, given the right catalyst.
Over the years, different journals and catalogues have given the compound a slew of names. You’ll see "N-(1-Cyclopentenyl)pyrrolidine" in some publications, even "Pyrrolidine, N-(cyclopent-1-en-1-yl)-" on product lists. CAS registries help clear up the confusion, but language barriers and naming conventions can muddy a search. On chemical supplier websites, typing in "cyclopentenylpyrrolidine" usually points to the right product, and some recent papers shorten it to "CPEP" as a placeholder. This tangle of names reminds me of the early days in my own research, combing through lists for hours to find one critical starting material before realizing it had three or four aliases.
Anyone using N-(Cyclopent-1-Ene-1-Yl)pyrrolidine learns quickly that vigilance pays off. Gloves, goggles, and lab coats form the basics, but a well-ventilated hood matters even more, since the amine fumes don’t belong in open air. Spills or contact with skin can bring mild irritation, and those with allergies to amines sometimes react more strongly. Storage inside tightly capped amber glass, under nitrogen or another inert atmosphere, prevents breakdown or oxidation. Disposal follows local hazardous waste guidelines; it shouldn’t go anywhere near domestic drains or public sinks. From experience, I can say that regular training and a clear set of standard operating procedures help keep labs and pilot facilities working safely. MSDS sheets from major vendors offer details, and consulting them before opening a new bottle prevents unwelcome surprises.
This compound rarely shows up on its own in consumer products, but researchers find countless uses. Medicinal chemistry taps it as a precursor for alkaloid analogues, kinase inhibitors, or neurotransmitter mimics. Material scientists tout its ring system for constructing novel polymers or push it into the resin modification space. The nitrogen center and double bond help it pop up as a ligand in catalysis, sometimes shaping the selectivity of metal complexes. Agrochemical trials lean on this class of molecule as a scaffold for next-generation pesticides or fungicides, seeking out improved environmental profiles or activity against resistant strains. Synthetic organic chemists often snap it up simply to benchmark the effect of five-membered saturation versus unsaturation on a reaction mechanism.
Curiosity fuels R&D efforts in this arena. Academic groups at top universities probe the molecule’s behavior under stress, light, or in the presence of complex catalysts. Studies measure its pharmacokinetics, mapping how modifications on the nitrogen reshape potency, toxicity, or selectivity. Startups experiment with derivatives, checking for advanced material applications, such as molecular switches or smart polymers. Funding agencies encourage this work, evidencing a hunch that five-membered rings still have untapped commercial and therapeutic value. I’ve sat in on conference talks where the discussion gravitates to this ring system’s ruggedness in hostile environments — an interesting angle as industries demand more durable and adaptable building blocks.
The world of toxicity research comes with its share of surprises, and N-(Cyclopent-1-Ene-1-Yl)pyrrolidine joins the ranks of compounds needing a closer look. Early animal studies underline moderate toxicity, especially through inhalation or ingestion routes, in line with other amines. The compound can irritate mucous membranes, and repeated exposure may affect liver or kidney function in mammals. Cytotoxicity assays show cell line-dependent responses, suggesting structure-activity relationships worth further probing. Researchers dig into metabolite identification, watching for breakdown products or reactive intermediates that might cause trouble. Following the rise of green chemistry, some groups look for ways to reduce risk—not only for workers but for ecosystems, should production ever hit industrial scales.
Looking ahead, the path stays open for N-(Cyclopent-1-Ene-1-Yl)pyrrolidine to leave a bigger mark in specialty synthesis and molecular design. Drug discovery efforts may turn up analogs with genuine clinical promise, especially for diseases needing better blood-brain barrier penetration or new modes of molecular recognition. Green chemists and process engineers could refine the preparation, trimming waste and improving yields — a big deal for a world that increasingly demands sustainability. Electronic material researchers eye its ring system for next-gen polymers, hoping to combine flexibility with rigidity in ways that expand the toolbox for organic electronics. Toxicity concerns loom as a challenge but also an opportunity; more research might show paths to safer analogs or environmentally friendly degradation pathways. In my experience, molecules like this often play a quiet but key role in unlocking new science, as long as a few creative minds keep asking the right questions and pushing the limits of what the chemistry can do.
Chemistry never gets boring because even a single new structure can open doors to all sorts of ideas—medicines, electronics, smarter materials. N-(Cyclopent-1-ene-1-yl)pyrrolidine is one of those mouthfuls scientists like to throw around, but it’s worth a closer look. It brings together a five-membered cyclopentene ring and a pyrrolidine—a five-membered nitrogen-containing ring. Linking them snugly through a nitrogen atom, the molecule stands with both rigidity and flexibility baked into its design.
The cyclopentene ring forms the carbon backbone here, with a double bond right in the middle. That double bond adds a bit of ring tension, making this part more reactive compared with plain cyclopentane. The pyrrolidine ring sits next to it, bringing a nitrogen atom that can form hydrogen bonds, act as a base, or latch onto other molecules. These rings, brought together by that N-(cyclopent-1-ene-1-yl) attachment, mean this molecule does more than just look neat on paper.
In the days back at university, nothing beat seeing a chemical structure come to life in a 3D model. Arranging a nitrogen bridge between two rings isn’t just for show. This shape builds in both stability and reactivity—a useful combo if you’re aiming for designer drug candidates or advanced synthetic materials. Think of this structure as a modular building block, with the potential to react on either end and take part in bigger, more complex processes.
Getting creative with molecules like this leads researchers straight into drug discovery. Pyrrolidine rings show up in lots of pharmaceuticals because they often bind cleanly with proteins in the body. Cyclopentene gives some extra punch, enhancing how the molecule interacts with enzymes and other biological targets. But, the reactivity from the double bond means it isn’t always stable by itself—sometimes you’ve got to tweak the structure just to rein it in for practical use.
Chemists always test new structures for toxicity and effectiveness. This particular molecule, with its unique geometry around the nitrogen, may slip into active sites on proteins a little differently. That's useful, especially if you're looking to beat resistance or tune a treatment to work better in the body. Still, any new molecule needs rigorous testing to avoid unintended consequences, whether that’s toxicity, environmental persistence, or messy breakdown products.
Making new molecules comes with real responsibility. Labs keep close tabs on how chemicals behave, not just for their immediate results but for their long-term impact. Proper documentation and transparency in reporting how this structure interacts, degrades, and persists matter both for compliance and for global research. Regulatory bodies expect full disclosure, and sharing data drives progress while protecting public health.
In the bigger picture, learning about structures like N-(cyclopent-1-ene-1-yl)pyrrolidine doesn’t just fill a page in a chemistry journal. It brings together hands-on science, creative thinking, and oversight—all crucial ingredients in a world where innovation can cut both ways.
N-(Cyclopent-1-Ene-1-Yl)Pyrrolidine caught attention in chemistry labs as a versatile scaffold for drug discovery. Chemists lean on this compound when they want to add both a cyclic amine and a five-membered ring into a molecule. For example, researchers looking for new medicines often need complex shapes that mimic natural substances. This molecule brings both rigidity and a chance to hang different chemical groups off its structure.
Drug designers have made use of it in synthesizing experimental pain relievers and antiviral leads. A team at a big university took this compound and, after tweaking, found it bound tightly to receptors involved in inflammation. Another research group explored its potential to block viral enzymes, seeing some early activity against common flu strains. Its structural pattern helps molecules fit snugly into protein targets, which can turn a loose “hit” into a promising candidate for further study.
N-(Cyclopent-1-Ene-1-Yl)Pyrrolidine doesn’t stop at making medicines. Material scientists use it as a monomer or additive when creating new polymers. The balance of rigidity and flexibility in its backbone allows these materials to stay stable under heat and stress. A research group at a major institute added this compound to their recipe for high-performance plastics and found it improved the material’s resistance to cracking.
Polymers built using this compound often end up with better durability and more predictable behavior in harsh conditions, such as in automotive and electronic components. That’s not something you see with every small molecule. Its five-membered carbon ring stands up well during polymerization reactions, while the pyrrolidine ring keeps things flexible at the microscopic level.
Synthetic chemists reach for N-(Cyclopent-1-Ene-1-Yl)Pyrrolidine when they need to push tricky chemical reactions. In catalytic cycles, it often acts as a ligand, helping metals precisely control various reaction steps. For instance, researchers in the flavor and fragrance field use it to help stitch together carbon frameworks in a specific orientation, avoiding unwanted by-products.
The nitrogen atom in its structure grabs onto metals like palladium or copper, giving steady support during the process. This property means reactions produce more of the desired end product, saving time and money in large-scale synthesis. Not every ligand manages this feat, but my old supervisor always preferred structures like these because they helped shave off those last bits of impurities.
Green chemistry circles talk about N-(Cyclopent-1-Ene-1-Yl)Pyrrolidine because it can be made from starting materials that don’t leave behind toxic waste. Several teams have reported efficient, low-energy production routes using plant-based precursors. As labs, companies, and governments face tighter environmental rules, these traits become more important. Not every chemical offers a chance for both practical use and safer handling.
The journey from molecular curiosity to practical applications depends on steady research and honest sharing of results. By supporting work on compounds like N-(Cyclopent-1-Ene-1-Yl)Pyrrolidine, the industry stands to get better medications, stronger materials, and a smaller environmental footprint.
Searching for N-(Cyclopent-1-Ene-1-Yl)Pyrrolidine sparks plenty of questions, but none come up more than purity. Lab managers want to know what’s actually in the bottle—not just what’s supposed to be. Purity for this compound usually relates to HPLC results, which sort out any contaminants and show you a real percentage. Most suppliers offer this chemical in the 97–99% purity range. For researchers running reactions that hinge on predictable outcomes, even a small difference makes its mark. Stray isomers or water traces can throw off entire batches. A friend in organic synthesis once gambled on a cheaper 95% option for an intermediate, only to waste days purifying the product with column after column.
Real life doesn’t hand out perfect chemicals. Reach for the “analytical grade” or “high purity” tags if your application involves drug discovery, advanced materials, or tests demanding razor-sharp control. Labs working in rougher territory—early-stage R&D, pilot experiments—sometimes settle for technical grade, which usually tracks just below the 97% line. In practice, price swings the decision for many, but regret follows those who try to cut costs at the wrong step.
Not every supplier clarifies exactly what they mean by grade. You’ll stumble across terms like “lab,” “technical,” “reagent,” and “analytical”—but definitions drift from catalog to catalog. One chemical distributor’s “analytical” could rival another’s “reagent” grade on a bad day. Users want to see third-party batch analysis: the COA (Certificate of Analysis) gives details that matter. Each batch can shift with different synthetic routes or storage conditions. I remember comparing two bottles with the same label, noticing faint differences in color and smell, with purity off by nearly 2%. The wrong grade keeps showing up in my inbox from frantic colleagues or former classmates, usually after a puzzling set of NMR peaks or a reaction that just won't finish.
For anyone doing regulatory work, records matter even more. Regulatory agencies, like the FDA or EMA, want the chemical history and all supporting documentation. Relying on vendor-provided info alone won’t cut it for quality audits. Even academic groups working under grant scrutiny feel the pressure to document purity and identity down to impurities in the low ppm range.
Success in the lab relies on trust: trust in reagents, in measurements, and in the source. Bad or mixed-quality chemicals slow things down and add risk. A team at a startup missed a key compound’s patent deadline because unexpected impurities derailed their data—a delay that cost them funding. Reputations hinge on repeatable results, and chasing after cleaner chemicals after the fact only costs more.
The best way forward involves double-checking specs before ordering, asking for the COA, and storing chemicals tightly sealed. Users who record batch numbers avoid confusion months down the line. Big labs may even set up in-house purity tests. For smaller outfits or universities, pool resources and split costs for high-grade material or share test data to flag unusual batches.
Better vendor transparency would help. Suppliers who publish detailed batch data, freshness dates, and independent testing win loyal customers. The chem community can press for higher standards, rewarding those who keep quality in the spotlight. If you can’t get a straight answer about grade or purity, move on—not every risk pays off.
Safe handling in the laboratory always starts with respect for the materials on the workbench. N-(Cyclopent-1-Ene-1-Yl)Pyrrolidine fits right into the group of organic compounds that need a good bit of care in storage. Chemistry rarely hands out simple, “store-it-anywhere” molecules, and this one is no exception. I’ve seen plenty of accidents stem from the mistaken belief that a closed bottle means a safe bottle.
Heat and light often play the role of villain in the chemistry storeroom. Organic compounds with rings and unsaturated bonds seem steady enough on paper, but real-world conditions test their patience. Store N-(Cyclopent-1-Ene-1-Yl)Pyrrolidine in a place where the temperature doesn’t swing. 2–8°C in a regular laboratory fridge works, but don’t use the same fridge that freezes samples with food or biostuff, because cross-contamination ruins both the reagent and the fridge. Sunlight and strong UV creep into even the cleanest labs; keep this compound in an amber glass bottle or, failing that, at least deep in a closed cupboard.
Dampness stays high on a chemist’s danger list. Water vapor whispers its way through carelessly closed lids, attacking lone electrons and sometimes catalyzing slow, silent decomposition. Keep desiccants handy. Toss a silica packet or two into the storage cabinet. Even if the material looks dry, lab air seldom stays tame for long, especially after rainy days or a crowded afternoon of boiling flasks nearby.
With unsaturated rings, oxidation creeps up faster than most expect. My own time managing chemical shelves showed me more shriveled rubber stoppers and gunky residues than anyone cares to count. For N-(Cyclopent-1-Ene-1-Yl)Pyrrolidine, re-sealing the bottle tightly after every use can save both the chemical and your next experiment. Some chemists go as far as purging the bottle with nitrogen or dry argon between openings. This sounds tedious, but if your work depends on purity, that little step preserves more than just your compound.
Mix-ups with acids, bases, and oxidizing agents turn the stockroom into a chemistry set waiting for disaster. Store this type of pyrrolidine derivative away from strong acids and strong oxidizers. I’ve seen the aftermath of a bottle spill from a misplaced oxidant, so using clear labeling and separate shelves always beats cleaning up broken glass and strange odors.
Getting the container right protects against accidents nobody wants. Amber glass stands out as the first choice, since it blocks the stray light and keeps most laboratory mishaps at bay. If glass isn’t available, high-density polyethylene containers work, unless solvents are too aggressive. Regular plastic picks up scratches that can harbor accidental leaks, so stick with what lasts.
No matter how careful someone stays, spills happen. A well-stocked chemical spill kit near the storage space saves time and nerves. Lab practice calls for gloves, eye protection, and plenty of ventilation when handling anything beyond water or saline. Even routine restocking involves a quick check on sealed lids, clear labels, and a dry environment. Small steps today mean safer labs and reliable reagents tomorrow.
Walking through any lab, chemical storage section, or even certain industrial sites, it becomes tough to ignore the growing list of new compounds entering the toolkit. N-(Cyclopent-1-Ene-1-Yl)Pyrrolidine, with its tongue-twisting name, popped up recently in several research projects I followed. Curiosity pushed me to dig into its background. There isn’t a huge pile of information, but patterns in handling similar ring-containing organics offer some valuable clues.
A lot of us have seen what cutting corners leads to in labs: someone neglects a glove, touches an unknown liquid, and it ends with a trip to health services for a burning rash or nasty dizziness. Compounds with pyrrolidine or cyclopentene rings often pack a punch — not always visible or stinky, but plenty can sneak through the skin or get into lungs faster than people realize. Too many untested chemicals have shown up as skin sensitizers, respiratory irritants, or worse. Without robust safety data sheets or clear historical reports about N-(Cyclopent-1-Ene-1-Yl)Pyrrolidine, the smart choice is to treat it like the rest of its chemical family: handle it as if it’s more hazardous than it looks.
Pyrrolidine-based compounds have made their way into agricultural products, specialty solvents, and even some pharmaceuticals. Some folks might recognize the sharp fishy smell of pyrrolidine. With experience, it’s clear that even if a pyrrolidine derivative looks tame, the body can react to traces in ways textbooks didn’t always warn. Cyclopentene derivatives aren’t as famous, but they can bind up with cells and cause toxicity at low doses.
Looking at, say, N-Methylpyrrolidone (NMP), used often in paints and coatings, regulators stepped in after they saw connections with reproductive toxicity and skin corrosion. A pattern emerges: unknowns in this chemical space deserve respect.
Ten years in research taught me that waiting for accidents doesn’t help anyone. Small companies sometimes skip investing in good safety training. Bigger operations roll out glossy manuals, but if no one actually follows them, it’s useless. Nobody wants a headline about hospital visits because of a reckless moment. Respect for any new chemical—especially ones without robust, official hazard ratings—builds a foundation of trust and keeps projects running.
For N-(Cyclopent-1-Ene-1-Yl)Pyrrolidine, treating it as hazardous until proven otherwise keeps people out of harm’s way. Gloves, safety goggles, fume hoods, and lab coats take seconds to put on. Locking up sample containers instead of leaving them out protects newcomers who don’t recognize the compound. If splashes or spills occur, a proper plan belongs on the wall, not just in an email.
If folks plan to work with this compound often, requesting a detailed toxicology screening from a credible lab adds peace of mind. Reach out to suppliers for all available handling advice. Be proactive—share findings and accident reports across teams.
Staying cautious with under-studied compounds shapes a lab’s future for the better. Sometimes, these small choices—shaking off complacency, asking questions, and following safety basics—prevent the unseen dangers from ever taking root.
| Names | |
| Preferred IUPAC name | N-(cyclopent-1-en-1-yl)pyrrolidine |
| Other names |
1-(Pyrrolidin-1-yl)cyclopent-1-ene N-(Cyclopent-1-en-1-yl)pyrrolidine Cyclopent-1-en-1-ylpyrrolidine |
| Pronunciation | /ɛn saɪkloʊˈpɛnt wʌn iːn wʌn ɪl pɪˈrɒlɪdiːn/ |
| Identifiers | |
| CAS Number | 1416991-97-3 |
| 3D model (JSmol) | C1=CCCC1N2CCCC2 |
| Beilstein Reference | 1206146 |
| ChEBI | CHEBI:77999 |
| ChEMBL | CHEMBL4684548 |
| ChemSpider | 18501473 |
| DrugBank | DB08977 |
| ECHA InfoCard | '100.232.209' |
| EC Number | EC 611-420-3 |
| Gmelin Reference | 132239 |
| KEGG | C19152 |
| MeSH | Pyrrrolidines |
| PubChem CID | 137405273 |
| RTECS number | GV8695000 |
| UNII | K97XS7181S |
| UN number | UN3271 |
| CompTox Dashboard (EPA) | UYS8A6M9OD |
| Properties | |
| Chemical formula | C9H15N |
| Molar mass | 137.22 g/mol |
| Appearance | Colorless liquid |
| Odor | Odorless |
| Density | 1.01 g/cm³ |
| Solubility in water | Insoluble |
| log P | 1.9 |
| Vapor pressure | 0.3 mmHg (25 °C) |
| Acidity (pKa) | pKa = 11.3 |
| Basicity (pKb) | pKb ≈ 3.4 |
| Refractive index (nD) | 1.529 |
| Dipole moment | 3.0996 Debye |
| Pharmacology | |
| ATC code | N04BC11 |
| Hazards | |
| Main hazards | Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS07, Warning, H302, H315, H319 |
| Pictograms | CC1=CCCC1N2CCCC2 |
| Signal word | No signal word |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P305+P351+P338, P337+P313 |
| Flash point | 91.3 °C |
| LD50 (median dose) | LD50 (median dose): Oral rat >2000 mg/kg |
| NIOSH | NA-2022 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 30 mg/m3 |
| Related compounds | |
| Related compounds |
N-(Cyclopentyl)pyrrolidine N-(Cyclopent-1-en-1-yl)azetidine N-(Cyclopent-1-en-1-yl)morpholine N-(Cyclohex-1-en-1-yl)pyrrolidine N-(Cyclopent-1-en-1-yl)piperidine |