Chemists have chased the idea of marrying morpholine’s flexibility with unsaturated hydrocarbons since the mid-20th century, and N-(Cyclopent-1-en-1-yl)morpholine grew out of experiments aimed at expanding nitrogen-containing heterocycles for pharmaceuticals and materials sciences. The journey began with curiosity about how attaching a cyclopentene ring to morpholine’s nitrogen backbone influences reactivity and overall function. Early synthesis routes—often messy and inefficient—suffered from limited yields and selectivity. Researchers in the 1970s turned up the heat, quite literally, looking for better catalysts and solvents. This compound gained traction in academic circles, especially among labs in Europe and North America, where morpholine derivatives carried expectations for both agricultural and pharmacological use. Documentation became more systematic in the 90s; teams started publishing improved prep routes and early tests for its chemical behavior. Open sharing of synthesis methods and safety data, pushed by public scientific norms, made this molecule accessible for more than just a handful of labs. Now, you won’t find it catalogued like aspirin or caffeine, but it holds a steady spot in niche research.
Strip away the jargon, and N-(Cyclopent-1-en-1-yl)morpholine stands as an organic compound created by attaching a cyclopentene ring to the nitrogen atom on the morpholine ring. The compound carries no star-power for mainstream chemistry, but its unique structure fills a gap—offering access points for modification, conjugation, and reactivity study. Instead of bulk production, manufacturers make small, targeted batches for research. Each bottle, often amber-glass to protect from light, comes clearly labeled, usually stored away from direct heat and humidity. Prices run higher than simple morpholine or cyclopentene derivatives due to the specialized synthesis and limited demand.
N-(Cyclopent-1-en-1-yl)morpholine usually appears as a faintly yellow oily liquid at room temperature, with an odor that reflects its morpholine roots—somewhere between sweet and amine-sharp. This compound resists dissolving in water, turning instead to common organic solvents such as chloroform, dichloromethane, and ether. Its boiling point sits south of 200°C, with a modest melting point, reflecting the influence of both the morpholine and the unsaturated cyclopentene components. Chemically, it carries a double bond inside the cyclopentene ring, which acts like a reactive handle for later modifications. The nitrogen atom provides another reactive site, especially for acylations and alkylations. Molecular weight calculations and NMR spectra confirm its identity; the compound’s IR signature gives clear peaks for C=N stretches and C-H bending from both rings.
Look through technical labels, and suppliers list purity, often above 95%, with reference to HPLC or GC trace analysis. Safety sheets go out with every shipment, marked with hazard pictograms, GHS statements, and supplier-specific lot numbers for traceability. Labs assign catalog numbers to maintain consistency, and data sheets break down shelf life, recommended storage conditions—usually cool, dry, away from incompatible chemicals. Physical constants such as boiling point, melting point, refractive index, and density support researchers in confirming compound identity and planning reaction conditions. This focus on detail stems from the compound’s use in sensitive synthetic applications; even small changes in purity or storage can alter experimental outcomes.
Synthetic routes draw inspiration from basic organic transformations. Most start with cyclopentenone as a building block, converting the carbonyl to an imine or an activated intermediate, then introducing morpholine as the nucleophile. Acid or base catalysis speeds up the process; careful distillation removes by-products or excess solvents. Some chemists add light or catalyst-driven tweaks, pursuing either higher yields or cleaner product—sometimes both. A typical setup includes a round-bottom flask, magnetic stir bar, and a nitrogen atmosphere to fend off unwanted oxygen. After the reaction, organic extraction and column chromatography polish off the crude mixture. Multiple rinses with brine and drying agents precede the final concentration and, if needed, vacuum distillation for further purification.
The cyclopentenyl double bond brings a world of reactivity: hydrogenation tames it, while epoxidation opens fresh routes to diols and related structures—each with its own role in fine-tuning molecular function. The morpholine nitrogen, stubborn only under extreme pH, welcomes alkylation and acylation. Chemists looking to build more complex scaffolds might use cross-coupling reactions, tapping into the electron-rich double bond. Halogenation offers another modification angle; introducing a bromine or chlorine atom on the ring can steer subsequent reactivity. Academic labs have even probed Diels-Alder reactions using the cyclopentene ring, looking for ways to expand or rearrange the molecule into new heterocyclic systems.
Walk through chemical catalogs and you’ll find this compound listed under a variety of names, which often trip up anyone without a keeper’s memory for IUPAC naming conventions. Commercial listings call it N-Cyclopentenylmorpholine, Cyclopentene-Morpholine, and sometimes N-(1-Cyclopentenyl)morpholine. Older literature swaps out names in favor of shorthand or catalog codes, depending on the supplier or country. Its structure can be summarized by its dual backbone: a morpholine ring attached to the five-carbon unsaturated cyclopentene. Researchers can cross-check synonyms in chemical databases to avoid confusion over isomers or closely related structures.
Working hands-on with N-(Cyclopent-1-en-1-yl)morpholine brings a set of predictable, but serious, precautions. Skin and eye irritation top the list of risks, and its vapor can irritate mucous membranes. Fume hoods protect against inhalation. Personal protective equipment goes beyond just gloves; safety goggles and full lab coats become daily wear. Standard chemical hygiene protocols keep spills manageable—absorbent pads, neutralizing agents, and properly labeled waste containers cut accident risk. Emergency showers and eyewash stations remain essential in every lab that stores or uses this compound. Waste disposal stays in line with regulations governing amines and reactive hydrocarbons, requiring treatment as hazardous chemical waste rather than safe-for-the-drain discards. Training new researchers in up-to-date SDS (Safety Data Sheet) content can mean the difference between minor inconvenience and major lab incident.
This compound doesn’t appear in the list of everyday products, but its unique structure opens doors in medicinal chemistry, agrochemical research, and synthetic methodology development. Pharmaceutical teams experiment with N-(Cyclopent-1-en-1-yl)morpholine as a building block for bioactive molecules, probing for effects that hinge on its dual-natured core. Crop protection research teams study potential antifeedant or insecticidal activities, although most work stays in early-stage screenings. The compound’s unsaturated ring and secondary amine function as test beds for new reaction types, giving academic researchers a playground for reaction optimization and mechanism study. Material scientists, still in the early days, consider this structure for its film-forming and adhesive properties, given the inherent stickiness of both morpholine and unsaturated carbocycles.
Laboratories around the world view N-(Cyclopent-1-en-1-yl)morpholine through two broad lenses: structure–activity relationship studies and synthetic methodology innovation. Medicinal chemists make subtle modifications to find leads for new medicines, running computer simulations, then wet-lab screens, searching for something that shapes biological targets. Synthetic chemists borrow the molecule’s dual functionality to explore catalyst development, aiming for new ways to transform old reactions. Not every experiment brings positive results—sometimes, a tweak destroys all activity. Yet, every negative result shapes the next approach, teaching chemists about bond stability, steric hindrance, and ring-opening potential. Cross-disciplinary collaboration stands out; a pharma-focused team shares data with synthetic or analytical chemists, spurring projects that rarely run solo in today’s research world.
Studies on the toxicity of N-(Cyclopent-1-en-1-yl)morpholine remain thin but instructive. Early work checks the fundamental toxicity in cell lines and rodent models, looking for acute, sub-acute, and chronic effects. Results suggest moderate hazards, such as skin irritation and sensitization, but so far stop short of revealing any major carcinogenic or mutagenic risk. Like many morpholine derivatives, researchers still worry about long-term exposure, especially since morpholine itself carries caution due to liver and kidney effects at high doses. Ecotoxicology studies stay rare, but preliminary data show slow breakdown in soil and water, flagging a need for care in disposal to avoid long-term buildup. It’s a reminder that even chemicals with little direct consumer use still deserve watchful stewardship, both in lab environments and for the planet at large.
Chemical innovation depends on keeping doors open, and N-(Cyclopent-1-en-1-yl)morpholine holds potential as research climbs past early trials into more systematic application. As computers grow smarter at predicting molecular interactions, this compound—rich with reactive sites—will likely draw more interest for tailored modification and scaffold development. In materials science, its combination of chemical stability and reactivity sits right where researchers hunt for next-generation adhesives, coatings, or drug delivery systems. Greener synthesis methods loom on the horizon as labs try to reduce waste and energy use. Regulatory demands will define just how easy—or hard—it will be to move from lab curiosity to practical product, especially as safety data expand beyond the basics. Chemistry students today may find themselves handling this compound as part of coursework in reaction mechanisms or safety-in-design—a vivid example of how molecular research rarely stands still.
It rarely gets much press, but N-(Cyclopent-1-En-1-Yl)Morpholine ends up carrying a lot of weight in the world of research chemistry. You won’t spot it in your kitchen cabinet, and most people with a day job outside the lab haven’t heard its name. It earns its keep mainly as a building block—helping chemists put together much larger, more valuable molecules for testing or commercial routes. This is a point where chemical creativity and practical engineering come together, which matters if you care about new medicines or materials.
From experience, work on novel pharmaceuticals always starts with experimentation. Trying a hundred paths, failing most times, learning just a little each round. Compounds like this one act as scaffolding. Chemists set up a backbone, like the cyclopentenyl group, then tack on morpholine for its handy nitrogen. That small tweak gives later researchers a base for testing or improving biological effects. Sometimes, a small change makes a drug more stable or easier for the body to absorb.
Real breakthroughs don’t happen unless you can try lots of variants quickly. N-(Cyclopent-1-En-1-Yl)Morpholine shows up as a kind of modular block. Pop it in, adjust a few atoms, and you get a whole new set of properties to study. Teams chasing the next antiviral, cancer therapy, or agricultural agent know this pain and hunt compounds that slot into their toolkits.
Lab notebooks fill with pages devoted to fine details of how nitrogen atoms affect the bigger chemical structure. Besides looking at medical uses, researchers evaluate these molecules for new materials. The morpholine ring can increase flexibility or improve how certain plastics behave under heat or exposure to UV light. There’s a real push to develop materials that last longer, resist degradation, or do new things, like self-heal or conduct electricity better. Molecules like this one don’t headline those breakthroughs, but they absolutely start the chain reaction.
Sitting at my desk, reviewing compound requests, safety always forces a pause. Scientists work with strong guidelines. Chemical houses require clear documentation before they ship small batches to legitimate research labs. There’s a risk if these building blocks end up in the wrong hands, or if they accidentally slip into the consumer market without strict controls. Regulators have put rules in place, checking on suppliers and end users, especially as new research comes out about possible unintended effects or toxicities of untested derivatives.
At the same time, over-regulation can slow progress. I remember frustrated colleagues losing weeks to paperwork, waiting for permission to use a single new compound. The risk of accidents or misuse gets a lot of coverage, but the bigger worry is losing the chance to discover better drugs because the necessary building blocks stay locked away.
A balance keeps progress moving. Trusted suppliers and open databases help ensure the right people, with the right training, get legal access. Governments can improve tracking systems while reducing delays for qualified research. Open communication about potential short- and long-term effects encourages everyone to stay alert without choking off the cycle of trial and discovery.
N-(Cyclopent-1-En-1-Yl)Morpholine doesn’t turn heads, but for the teams chasing tomorrow’s breakthroughs, it quietly provides what they need to keep the search alive.
Anyone working with N-(Cyclopent-1-En-1-Yl)Morpholine needs to respect that even obscure chemicals can bring real risks. Some colleagues look at novel compounds and see curiosity first, risk second. That mindset turns dangerous quickly. Chemicals like this don’t need a long history to cause headaches—sometimes literally. My own early lab days taught me through nose-burning trial and error that it's far easier to prevent an accident than explain one.
Let’s take the basics: this compound belongs to the morpholine family, which means it carries some punch. Liquid or vapor, the amine-like nature means it can irritate eyes, skin, and airways. So, nobody should treat it like harmless lotion. Getting lazy with gloves or neglecting eye protection brings unnecessary risks. Even seasoned chemists get caught off guard once; after that, most reach for safety glasses without a second thought.
Standard nitrile gloves handle most spills, but double-gloving can help in case of punctures. A lot of folks underestimate splash risk—one slip, a droplet lands on skin, and irritation follows. Safety glasses or, better, a face shield block out the real threat of eye exposure, which can turn a workday sour fast. Lab coats keep splashes off street clothes and prevent accidental transfer to public spaces.
A properly ventilated fume hood matters. Respiratory irritation can sneak up, especially in shared labs where open bottles—or just slow evaporation—add up. One forgotten vial left overnight, and the next morning you walk into uncomfortable symptoms.
Pouring and weighing deserve real care. Even in a rush, take time to use a clean spatula and avoid direct breathing over open containers. Good habits pay off by keeping exposure down. Nobody wants to learn later about subtle chronic hazards; better to play it safe from the outset.
Waste elimination plays into long-term safety. Storing leftover chemical in robust, sealed containers—properly labeled—stops mistaken identity issues. I’ve seen what confusion over a mystery bottle can do: best-case, someone loses time or has to treat all waste as hazardous; worst-case, somebody mixes incompatible materials. Clear, legible labeling and dated logs mean future teammates won’t curse your name.
Spills will happen eventually, even for careful hands. Small amounts get absorbed with spill pillows or absorbent pads, scooped into sealed bags, and disposed of with chemical waste. Bigger spills need evacuation—don’t try to be a hero. Alerting lab mates and grabbing the eyewash or safety shower saves skin and vision. Posters next to sinks and doors help newbies remember the right steps under pressure.
Bottles stored on lower shelves or in secondary containment reduce knock-over risk. I learned to triple-check lids after a single bottle rolled off a cart and cracked years ago; lessons like that only need to happen once. A chemical-resistant tray can stop a mess from spreading across countertops.
No safety plan works alone. Open conversation makes a difference. Sharing what you’ve learned from real mishaps keeps colleagues on their toes. Regular reviews of the latest safety data sheets means no one falls behind on understanding what they’re handling. Over time, people new to the lab take safety as seriously as old hands do.
It’s easy to settle into routine in any workspace, but real safety comes from staying alert and humble. No chemical, no matter how routine tomorrow, deserves less attention today.
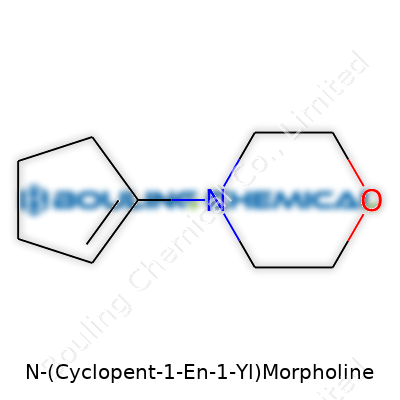
N-(Cyclopent-1-en-1-yl)morpholine isn’t a term you’ll run across at the grocery store, but its structure brings together familiar chemistry: morpholine, a ring made up of four carbons, one nitrogen, and one oxygen. It’s paired with a cyclopentenyl group, which is just a five-membered ring with one double bond. Bring these two together, and you’ve got C9H15NO as your molecular formula.
To picture its skeleton, the morpholine holds the core—a ring where nitrogen and oxygen give it some interesting properties: basicity from the nitrogen and polarity from the oxygen. The cyclopentenyl group connects at the nitrogen, making for a compound that’s more reactive than plain morpholine. Since morpholine derivatives see use in pharmaceuticals, agrochemicals, and even in corrosion inhibitors, it’s not just a random pairing. Chemists often explore these sorts of combinations to see how they tweak performance or binding.
Chemical structure determines what a molecule can do. With N-(Cyclopent-1-en-1-yl)morpholine, the cyclopentenyl ring brings in an unsaturated bond which, compared to a saturated cyclopentyl ring, gives a spot for further reactions. This means it could grab onto other atoms, making it a good intermediate if you’re aiming for more complex molecules. The presence of the morpholine ring, with its blend of nitrogen and oxygen, helps the compound dissolve in water while also staying stable enough to play well with both acids and bases.
From experience in the lab, rings make all the difference, both for how the molecule behaves and how tough it is to synthesize. The double bond in the cyclopentenyl piece isn’t just a niche feature—it’s a handle for adding on extra pieces, sometimes used in making drug candidates more potent or more selective. Mixing a cyclopentenyl ring with morpholine doesn’t show up every day in textbooks, but every novel structure adds to the toolkit for synthetic chemists and pharmaceutical developers.
It’s not always clear how a given compound will be used until someone puts it through its paces. Morpholine by itself turns up in fungicides, rubber accelerators, and pharmaceuticals—think of it as a Swiss army knife for chemists. Adding a cyclopentenyl group gives a new twist that researchers can use to target specific enzyme sites in the body or create materials that block corrosion. Traditionally, any molecule with both nitrogen and oxygen has the potential for creating hydrogen bonds, which helps it interact better with water or biological targets.
If everyone in the chemical enterprise had access to novel molecules with diverse functional groups, drug discovery and materials science would move faster. New structures let scientists find better candidates for fighting diseases or building more durable polymers. The combination of aromatic and saturated rings, as seen here, is only one way researchers blend reactivity and stability.
One practical problem with making N-(Cyclopent-1-en-1-yl)morpholine is getting the right cyclopentenyl group and attaching it cleanly to morpholine. Side products can form if the double bond reacts somewhere you don’t want. In the lab, this means extra purification steps, more reagents, and time. One solution is to use well-chosen catalysts that only attach the cyclopentenyl ring at the nitrogen, cutting down on wasted starting material and minimizing environmental impact. Green chemistry principles recommend less hazardous solvents and milder reaction conditions—this leads to fewer harmful leftovers and a cleaner, safer process.
To sum up real-life impact: new molecules like this expand our understanding of what’s possible with amine-heterocycle chemistry and fuel innovation in fields where molecular design matters, from medicine to manufacturing. Through careful structure analysis and synthetic method development, chemists keep opening the door to solutions for tough problems in health and industry.
Most labs and research teams place a huge value on knowing exactly what they’re working with. Purity tells you just how uncontaminated a chemical is. For N-(Cyclopent-1-En-1-Yl)Morpholine, you usually see 97% or sometimes 98% purity levels on the market. Anything less and you’re running a bigger risk of skewed results or batch inconsistencies. This kind of information makes life a lot more predictable when you’re carrying out tough analyses or process development.
I’ve learned—after several years working alongside both academic teams and process engineers—that a 97%-plus rating is the point where few folks worry about unexpected byproducts. It means you can follow protocols or instructions with less need for additional purification steps. For those running quality control or scaling up pilot projects, this level of assurance builds trust across the board.
Having confidence in purity also smooths out paperwork. A chemical with reliable certificates of analysis speeds up documentation, regulatory submissions, and export dealings. Smaller labs, who may lack pricey analytic gear, lean on supplier testing and transparent reporting. It saves time, avoids extra verification steps, and limits the headaches that come from chasing down suppliers with vague answers.
A chemical like N-(Cyclopent-1-En-1-Yl)Morpholine tends to arrive in a range of packaging, each geared toward a particular use. Most suppliers ship it in standard glass bottles or HDPE containers with 1-gram, 5-gram, and 25-gram options. Larger scale requests—say for early phase pharma or material science pilot runs—might bring 100-gram or 500-gram bottles. But the most common packs you’ll run into are 1-gram or 5-gram jars.
In my own experience, the 5-gram size hits the sweet spot. It covers enough ground for optimization work and small-batch syntheses, but you’re not stuck with excess that will languish on your shelf. Precious specialty chemicals don’t just have a price tag—they carry risks if mishandled or stored long term. Choosing the right size links directly to safety and the bottom line. Order too much, and you’re stuck with waste disposal costs or stability worries. Buy too little, and you stall out mid-project.
There’s also an environmental side to think about. Oversized packaging leads to chemical waste, and that’s trouble for everyone from university storerooms to big industry safety officers. Bulk packaging can make sense for experienced users who know they’ll use every drop, but for most, smaller increments lower hazards and cut down on unnecessary leftovers.
Plenty of folks overlook basic supply questions and wind up with bills for hazardous waste removal or project delays. More than once, I’ve seen teams forced to scramble because they couldn’t track down a second batch with matching purity. Some chemicals come and go from supplier catalogs, especially ones that aren’t household names. Reliable packaging sizes and clear purity certificates add a layer of predictability.
Suppliers improve trust if they can back up claims with full batch testing and up-to-date safety data. My own success working with niche chemicals often hinges on this sort of transparency. If the market drifts away from 97% and higher purity, or if packaging choices shrink, labs pay the price in wasted effort, money, and time.
Clear communication, better packaging choices, and predictable purity all help projects hit their mark. The more transparent suppliers become, the easier it is for real-world science to move forward safely and efficiently.
N-(Cyclopent-1-En-1-Yl)morpholine shows up in research circles and specialty chemical labs. Most people who handle this chemical know that its stability and safety depend on proper storage. Unlike household items, specialty reagents demand more than just a spot on the shelf; they require a routine and a method.
Every chemist learns early that the wrong temperature or humidity can turn a stable compound into a risky mess. From the shelves at school to the back rooms of manufacturing plants, stories float about ruined batches and dangerous spills. I've seen firsthand how a neglected bottle with an eroded cap ruined research work and left everyone scrambling. N-(Cyclopent-1-En-1-Yl)morpholine deserves respect and care because, like many morpholine derivatives, it's sensitive to moisture and air.
A well-ventilated chemical storage room with limited access offers the best starting point. Direct sunlight or changing temperatures cause compounds to degrade or react with their containers. Temperature matters most here: 2–8°C keeps this particular chemical safe for the long term, so a reliable laboratory fridge becomes a friend. Never keep volatile organics near open flame or heat sources. Humidity control counts as well; desiccators and silica packs help soak up stray moisture in the storage area.
Pairing N-(Cyclopent-1-En-1-Yl)morpholine with glass containers, especially those with tightly screwed lids lined with PTFE, adds insurance against air infiltration. Polyethylene and polypropylene containers work, but glass offers more protection for volatile compounds. Labeling matters: mark dates, concentrations, and hazards clearly because confusion still happens even in careful labs.
Shelf life often gets treated as an afterthought, but anyone who’s watched a project fall apart over an expired reagent learns to check dates first. Manufacturers typically recommend using pure N-(Cyclopent-1-En-1-Yl)morpholine within two years if kept between 2–8°C, sealed tight, and away from moisture. Stability tests back this window up—exposure to higher temps, direct light, or compromised containers shortens its usable time frame.
Most chemists track shelf life with routine inspections and periodic checks for discoloration, precipitation, or odd odors. Once you spot any of these changes, don’t risk the experiment or anyone’s safety. Discard the material following hazardous waste protocols; don’t dump or store questionable chemicals hoping for the best.
Routine matters more than luck. Review inventory logs every few months, rotate stock so the oldest bottles get used first, and resist the temptation to save money by hoarding old batches. If the budget allows, invest in temperature-logged fridges and alarmed humidity monitors; errors usually creep in when you least expect them. Proper chemical storage training for all staff prevents simple mistakes that lead to big problems.
Manufacturing teams, academic labs, and R&D facilities all run faster and safer with strong protocols. Chemistry isn’t only about mixing ingredients. It comes down to protecting colleagues, experiments, and equipment from preventable hazards. With N-(Cyclopent-1-En-1-Yl)morpholine, sound storage and vigilance help everyone avoid costly failures and dangerous surprises. In the end, respect for the material pays off well beyond the balance sheet.
| Names | |
| Preferred IUPAC name | N-(cyclopent-1-en-1-yl)morpholine |
| Other names |
N-Cyclopentenylmorpholine Morpholine, N-cyclopent-1-en-1-yl- N-(Cyclopent-1-en-1-yl)morpholine N-(Cyclopentenyl)morpholine |
| Pronunciation | /ɛn saɪ.kloʊ.pɛnt wʌn ɛn wʌl mɔːr.fəˌliːn/ |
| Identifiers | |
| CAS Number | 1416998-55-4 |
| Beilstein Reference | 1843304 |
| ChEBI | CHEBI:187870 |
| ChEMBL | CHEMBL3702047 |
| ChemSpider | 21301143 |
| DrugBank | DB08995 |
| ECHA InfoCard | 36e1bb60-d24d-4b9c-943a-bda965764160 |
| EC Number | EC Number: 696-195-9 |
| Gmelin Reference | 12659116 |
| KEGG | C10638 |
| MeSH | Cyclopentenes; Morpholines; Heterocyclic Compounds |
| PubChem CID | 164858803 |
| RTECS number | GQ3158000 |
| UNII | JIR6D7UU8F |
| UN number | UN2810 |
| CompTox Dashboard (EPA) | DTXSID70823795 |
| Properties | |
| Chemical formula | C9H15NO |
| Molar mass | 153.23 g/mol |
| Appearance | Colorless liquid |
| Odor | Odorless |
| Density | 1.04 g/cm³ |
| Solubility in water | Insoluble |
| log P | 1.82 |
| Acidity (pKa) | 5.5 |
| Basicity (pKb) | pKb = 5.93 |
| Magnetic susceptibility (χ) | -72.37×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.5220 |
| Dipole moment | 2.85 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 380.7 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | N05CM25 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P272, P273, P280, P302+P352, P305+P351+P338, P312, P321, P332+P313, P362+P364, P501 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | > 87.9 °C |
| Lethal dose or concentration | LD₅₀ (oral, rat) > 2000 mg/kg |
| LD50 (median dose) | LD50 (median dose): 220 mg/kg (rat, oral) |
| PEL (Permissible) | Not established |
| REL (Recommended) | 30 mg/kg |
| Related compounds | |
| Related compounds |
Morpholine Cyclopentene N-Substituted morpholines Cyclopentylamine N-(Cyclopentyl)morpholine |