Looking back through the industrial advances of the twentieth century, N-Cyclohexylbenzothiazole-2-sulfenamide carved out a place thanks to demand for tougher, safer, and more durable rubber products. As the tire industry stretched to meet automotive and aviation booms in the mid-1900s, early rubber accelerators lacked consistency and gave unpredictable results in vulcanization. Chemists set out to address these issues, and through deep study of the thiazole family, crafted this particular sulfenamide as a solution for precise, controlled rubber curing. Industry journals from the 1950s make regular mention of tire manufacturers reporting gains in resilience and aging properties. Within a few years, factories across Europe, North America, and Asia integrated this compound into their core compounding recipes, and by the 1970s, its manufacture fueled major segments of the chemical trade.
N-Cyclohexylbenzothiazole-2-sulfenamide usually goes under the trade name CBS or CZ. This accelerator forms a creamy to light gray powder or pellet, and sometimes you’ll find it as off-white crystals, depending on granulation by the supplier. Its primary job in the world has always focused on acting as an accelerator in the vulcanization of rubber, especially where resistance to heat and mechanical stress matter. The compound comes packed in double-lined bags, often with inert gas blanketing, since it needs shielding from ambient moisture and oxidants, both of which can chip away at its potency.
CBS gives off a slightly sulfurous scent, reflecting its thiazole and sulfenamide backbone. At room temperature, it stays solid, with a melting point typically around 96 to 105°C. You won’t catch this chemical dissolving easily in water—its solubility skews toward apolar solvents like benzene or chloroform. On the molecular level, a thiazole ring links up with a cyclohexyl group, forming a structure that proves stubborn against hydrolysis but more reactive when exposed to acidic or basic environments. This reactivity helps explain its accelerator role, giving consistent delay in the scorch period during rubber manufacture and then a sharp kick in curing speed as temperatures climb north of 140°C.
You can spot CBS on a technical data sheet emblazoned with a CAS number—95-33-0—plus grading labels for use in food-contact rubber, general-purpose industrial vulcanization, or formulations tuned for specific climates. European REACH-listed variants carry extended safety details, and every container must mark batch number, net weight, manufacturing date, and proof of purity, usually above 97%. Safety data sheets spell out maximum allowable dust concentrations and PPE requirements, alongside labeling elements outlining acute toxicity hazards and environmental handling rules. Manufacturers also include handling temperature guidelines, especially for contracts specifying bulk storage in warm climates.
Chemists typically make CBS by reacting 2-mercaptobenzothiazole with cyclohexylamine, often in the presence of sodium hypochlorite or hydrogen peroxide as oxidizing agents. This process relies on careful control of pH and temperature, since runaway exothermic reactions can compromise product quality or even pose safety risks. More than once in my own lab experience, I have had to intervene in pilot plant runs to adjust the feed rates of oxidant or slow stirring speed because the batch temperature began to drift. Poorly controlled synthesis can lead to high levels of impurities, such as residual benzothiazole or unreacted cyclohexylamine, which can skew curing results in downstream applications. So, for plant operators and quality control staff, tracking parameters in real-time becomes non-negotiable.
CBS can break down or react with a handful of functional chemicals commonly found in rubber recipes. Under high heat, the sulfenamide group snaps open, freeing a reactive sulfenyl species that forms bridges between polymer chains. This property powers its role as a delayed-action accelerator—a feature highly prized since it cuts down on premature curing, or “scorch”, in high-speed manufacturing. CBS can also undergo further transformations, such as sulfoxidation or amination, if producers want to tweak cure rates or compatibility with novel polymer blends. Scientists in technical service labs often run these modified products through bench trials, comparing rate of cure, modulus, and aging alongside conventional CBS, to figure out whether a new variant brings enough upside to justify the added costs.
On international cargo manifests, this chemical goes by different names: CBS, CZ, or N-cyclohexyl-2-benzothiazolesulfenamide. Retread and OEM tire plants might list it as Accelerator CBS. Some suppliers market proprietary blends that tuck in stabilizers or flow agents, but the backbone molecule stays the same. The industry’s common code—CBS—crops up on safety training posters, batching logs, and maintenance schedules. Having clear synonyms matters more than folks may guess, since shipments from China or India still get flagged in customs if the documentation doesn’t line up word-for-word with regulatory lists.
For all the technical benefits CBS brings, it can irritate the eyes, skin, and airways, especially as airborne dust. Factory protocols call for closed transfer systems and local exhaust ventilation near mixing lines. In my time overseeing compliance audits, line workers routinely cited the importance of good gloves—a split seam means direct exposure, and in concentrated form, CBS has enough toxicity to prompt chemical burns or long-term sensitization. Occupational exposure limits vary by region, but most plants target levels below 5 mg/m³. First aid kits in compounding rooms always include sterile saline for eye irrigation and SDS sheets for reference in case a spill occurs. Keeping the product locked tight and cool, away from oxidizers or acids, feeds into warehouse safety programs, and failure to do so has resulted in fines for more than one rubber compounding operation over the past decade.
Rubber tires alone account for a vast chunk of CBS demand, pushed by global trucking, aviation, and off-road mining. The compound’s unique chemistry means manufacturers can achieve long scorch safety during mixing and then rapid cure cycles once the vulcanization press hits its target temperature. Conveyor belts, gaskets, vibration mounts, and wire cable coatings round out other major uses. Medical device firms have looked at CBS-modified rubbers for specific applications where minimizing leachable organics matters, though stricter regulatory angles on migration and extractables have led to a search for even purer forms. In construction, you sometimes find CBS-formulated neoprene layers in concrete expansion joints, chosen for their ability to shrug off sunlight and temperature swings.
Research groups continue digging into tweaks of the CBS molecule. Some projects probe ways to shift cure profiles for next-generation elastomers, raising hopes for longer-wearing consumer products and lower energy use in manufacturing. There’s a chunk of contemporary research looking at blends that decrease nitrosamine formation—a health concern drawing attention across the European Union and US in recent years. I’ve seen university teams push modified CBS analogs in prototype tire treads, chasing after longer tread life and safer wet-weather grip. Advances in process analytics brought about by automated IR and GC-MS units allow for real-time monitoring, slashing waste versus older batch-off methods. The push for “greener” chemistry means that institutions explore biobased thiazoles, but for now, synthetic routes remain the norm.
CBS has attracted its share of health and safety scrutiny. Lab tests confirm that ingestion or prolonged inhalation at high doses can trigger liver and kidney effects in rodents. Animal studies prompted the setting of workplace exposure limits, and more recent in vitro work connects certain CBS breakdown products with cellular toxicity, especially in aquatic environments. Regulatory bodies across North America, Europe, and East Asia order regular monitoring of effluents from plants using CBS, since small amounts entering waterways can affect fish larvae or microinvertebrates. Firms in the sector sometimes fund independent review panels to probe the risk of contamination in consumer rubber goods. Despite many decades of use, researchers and public health teams keep tabs on population exposures near plants, and ongoing epidemiology adds to a growing global risk database.
Future directions for CBS hinge on advances in materials engineering and shifting health regulations. As more countries clamp down on nitrosamine precursors, research will keep pressing to further lower toxic byproducts. Tire and automotive sectors now look for recipes that allow higher mileage and lower rolling resistance—a quest that puts even finer demands on the accelerator’s purity and performance. The rise of electric vehicles, with their need for quieter and more durable tires, injects new urgency into improving rubber formulations. Advances in process control and analytical chemistry, like in-line NMR and mass-linked chromatography, offer promise for tight tracking of every production batch. Ongoing work in green chemistry may eventually yield fully biobased or recyclable CBS analogs, but the cost and technical hurdles remain steep compared with decades-old synthetic methods. Regulatory shifts, tighter supply chains, and global sustainability pushes mean that every actor in the rubber industry watches the CBS story develop—with chemists, engineers, and policymakers all working to balance cost, performance, and public safety.
Rubber might look simple. You can see it every day on tires, shoe soles, and conveyor belts at the grocery store. But rubber, in its raw form, has its limits. Tire producers have chased after ways to make rubber tough, resilient, and stable for a century. Here’s where N-Cyclohexylbenzothiazole-2-sulfenamide steps in.
This chemical speeds up vulcanization, which is the heat-driven process transforming mushy rubber into a durable material. The technical term for its job is accelerator. Instead of waiting hours, the chemical lets factories do the same in minutes. The result: strong rubber that keeps its shape after millions of rotations.
Tire factories love this because it helps with volume production. Nobody looks for slow methods on a tire assembly line. Time equals money, so shorter heating times mean lower costs. Plus, this accelerator produces rubber that resists heat, cracking, and chipping. For anyone driving long distances or carrying heavy loads, these qualities make a difference in road safety.
Rubber products made with this accelerator show up everywhere. The trucking industry relies on tire treads that stand up to summer heat. Bus companies need steady grip in rain and snow. The chemical’s reputation springs from these real demands. Tire shops rarely discuss the mix of chemicals tucked inside a black tire, but drivers feel that reliability in every trip.
The chemical also plays a role in protecting workers. Quick vulcanization means less exposure to fumes on factory floors. Studies on workplace safety keep getting attention. Factories upgrade their equipment and experiment with chemical mixes, but accelerators like this one usually stay in the formula. Some trade-offs get constant review, including cost, worker safety, and finished tire quality.
Every company wants to extend tire life and lower fuel consumption. Fewer blowouts on highways help avoid deadly accidents. But the industry has to manage chemical runoff and air emissions from rubber production. Regulatory agencies keep watch on how accelerators break down in the environment, especially as tires wear down and particles land in the surrounding soil or water.
Industry professionals talk about finding “greener” accelerators—formulas that give rubber the same strength without creating extra waste or sapling energy. It turns into a balancing game. Companies experiment with alternative chemicals or tweak factory processes. Some invest in filtration. Others help set standards for chemical safety and emissions.
N-Cyclohexylbenzothiazole-2-sulfenamide sticks around for good reasons—delivery vehicles last longer, buses offer safer rides, and the heavy tires on farm equipment get through harsh conditions. My own years seeing dozens of tire shops rely on trusted brands connect to the bigger picture: chemicals in the background shape the safety and performance millions depend on each day.
As tire designers ask more from their products—like better wear with less rolling resistance—these accelerators keep evolving. Honest labeling, worker safety, and responsible disposal aren’t abstract ideas. Factories and consumers both have a stake in each change. Progress rests on practical chemistry that listens to these needs and rolls forward, one mile at a time.
N-Cyclohexylbenzothiazole-2-Sulfenamide shows up in plenty of rubber plants as an accelerator in vulcanization. In my years working close to materials where the wrong environmental touch can turn trouble, the rules for storing this chemical seem less about paperwork and more about good sense. This compound isn’t just another powder sitting on a warehouse rack; treat it right or it won’t behave as expected in the mix, and nobody wants rework or worse, an unexpected safety incident.
Heat breaks down this sulfenamide. Once ambient temperatures pass 30°C, you roll the dice on shelf life. Consistent, cool, and dry beats out “close enough” conditions every time. High humidity means the powder can start to clump, making dosing a headache and raising the odds of slow degradation. Moisture also risks worse: chemical breakdown and increased sensitization risk for workers. If you’ve ever handled rubber chemicals on a sticky July afternoon, you’ll recognize how a bad storage call leaves you fighting lumps instead of making your batch run smooth.
Forget leaky bags or thin sacks. Sealed, sturdy drums, tightly closed after every single use, protect both the contents and the people working nearby. In real shop floors, I’ve seen the difference between materials fast-tracked to rework and those that still look fresh after weeks. It basically comes down to whether someone kept the air and dampness out from the start. After years in warehouses, my advice always sticks: shut the lid, check the liner, and keep containers off concrete when possible. Raise them up on pallets to keep moisture away from the base.
Direct sunlight cooks more than lunch—chemical breakdown ratchets upward if you park these drums anywhere heat or UV rays strike. Some folks treat storage space as an afterthought, slipping a pallet in the only open spot, even if that means parking it next to a south-facing window. Shed light, skip heat, and you lower hazards and protect performance, as well as consistency in compounding. Facilities built with true chemical storage in mind tuck these critical materials in the shadows, in spots with dependable airflow and climate control.
Ventilated spaces matter just as much as dryness. Let air grow stale, and you’ll find chemical odors settling in, pointing to slow leaks or spillage. Clean spaces make accidental cross-contamination less likely. Nobody wants mystery particles floating into sensitive chemicals. I keep work surfaces clear and always sweep up after handling powdered accelerators—this dials down the odds of residues spreading and helps everyone spot trouble before it grows. Every open bag, every spilled scoop is a small risk that adds up fast.
Never bank on luck. If a spill happens, I have always kept absorbent materials and dedicated disposal bins close by. Backup plans don’t cost much in time. Losses rise fastest after folks ignore simple precautions—systems for labeling, regular inspections, and a clear record of shelf life checks form the backbone of real chemical stewardship. Taking care of these details keeps accidents rare and helps production teams intervene before age or mishandling trade shelf life for performance headaches or, worse, safety events.
N-Cyclohexylbenzothiazole-2-sulfenamide, commonly called CBS, pops up most often in rubber manufacturing. Tire plants and rubber conveyor belt shops have workers who know the smell of it. CBS helps speed up vulcanization, that process that gives tires and other rubber goods their bounce and shape. Rubber chemistry is full of strange names, but this one always grabs attention for reasons beyond its spelling.
Facts from industrial hygiene put CBS into a spot people shouldn’t take lightly. The European Chemicals Agency classifies it as “Harmful if swallowed, irritating to skin and eyes, and may cause allergic skin reactions.” The substance can also break down and release toxic gases like aniline and benzothiazole when heated above 100°C, which happens a lot during rubber processing.
Labs conducted studies where rats breathed CBS dust. The animals developed lung irritation and showed signs of inflammation. Workers report skin complaints if their gloves leak, from redness to rashes that don’t go away fast. I spent summers in a tire shop, and we kept scrubbing arms and faces, but some days, itched from shoulder to wrist. You learn which soaps work best.
CBS is not as dangerous as many organic solvents or acrylates. Still, long-term exposure can build up into real problems. The allergic potential bothers health providers and employers. In regions where factories skip the protective gear, folks show up at clinics with dermatitis and, in rare cases, have trouble breathing.
The issues with CBS reflect a broader story in chemical safety. Most workers around these substances don’t get lab coats or fresh gloves every hour. Sprinkled on open mixers, CBS dust lands on skin, settles near eyes, and sometimes gets tasted because workers grab a sandwich before washing hands.
The good news comes from common sense and better tools, not complicated solutions. Factories that invest in closed mixing systems or solid-form CBS cut down on powdered dust floating in the air. Even a basic exhaust fan helps but swapping from powdered to pre-dispersed forms slashes the mess a hundredfold.
Personal protective equipment (PPE) makes a difference. Decent gloves and goggles beat the thin plastic stuff most people get handed. Rotate workers so one person isn’t on the mixing deck all day, every day. Post handwashing stations right at the entrance to work zones, not buried way in the back.
Regulators like OSHA and REACH don’t ban CBS outright. They require labeling for skin and eye risks, providing guidelines for safe handling. That keeps companies on their toes, since a surprise inspection can cost them hefty fines. In companies with good safety cultures, supervisors check on the crew, keep safety talks short, and reward people for speaking up if they see a spill or a leaky drum.
If a company considers switching to safer alternatives, the market now offers accelerators that trade some performance for lower toxicity. These alternatives tend to cost more, which can be tough for small operations, but the offset comes from fewer health claims and better morale on the floor.
Real progress arrives when workers trust the process, management puts cash into training and equipment, and nobody treats chemical dust as some ordinary nuisance. Manufacturers who know what’s in their raw materials and respect those hands mixing the batch stand to lose fewer hours to sick days and keep more people healthy.
N-Cyclohexylbenzothiazole-2-sulfenamide carries the CAS number 95-33-0. Many people in the rubber and chemical industries know this chemical by its other name, CBS, as it plays a big role as an accelerator during the vulcanization process. Vulcanization gives rubber its bounce, stretch, durability, and the resistance it needs to survive everything from sports shoes to car tires. CAS numbers, unique identifiers assigned by the Chemical Abstracts Service, help everyone in manufacturing, scientific research, and regulatory fields to make sure there’s no confusion about which chemical sits in front of them.
Having worked with rubber processing teams, I’ve seen how long product codes and complicated chemical names can trip up new staff. Confusing similar abbreviations, or skipping over a decimal in a CAS number, can lead to wrong orders that cost thousands. The CAS number, 95-33-0, eliminates mistakes and speeds up communication. I remember a colleague mixing up CBS with a different sulfenamide because the packaging label was faded. A quick double-check using the CAS number prevented a shutdown that day.
In environments with language barriers, teams from different parts of the world rely on these identifiers. A factory in Malaysia and another in Germany can both look up 95-33-0 in their systems and know exactly what to expect in the shipment. The system supports safety, traceability, and compliance with international regulations. Many countries have chemical inventories—like the U.S. TSCA and European REACH—that reference CAS numbers to enforce safety standards.
Mixing up chemicals isn’t just about losing raw material. It touches workplace safety. CBS can be hazardous if misused, potentially leading to skin irritation or allergic reactions. Governments track the storage and disposal of chemicals using CAS numbers to enforce environmental regulations. In audits, regulators always match inventory logs against these unique numbers to catch mistakes before they ripple into environmental or health hazards.
Major brands require documented proof of every ingredient in their supply chain. A single missing or wrong CAS number can lock up shipments at customs, slow down production, or—even worse—brand a company as careless in handling restricted substances. The consequences ripple beyond lost sales to include fines and loss of trust.
Clear labeling, robust inventory software, and ongoing staff training anchor chemical traceability. Regular audits and software that flags CAS number mismatches cut errors and improve reliability. Digital inventory systems allow staff to scan barcodes or QR codes tied to CAS numbers, greatly reducing the chance of human error. Some progressive plants print the CAS number right next to hazard warnings on every barrel and drum, making it visible from storage to workbench.
Production lines run best when small details like CAS numbers don’t get overlooked. Making the number 95-33-0 as routine as checking the date on a milk carton might seem obsessive, but in chemical safety, consistency always wins.
Rubber hits the road in every corner of modern life, and N-Cyclohexylbenzothiazole-2-Sulfenamide (a mouthful, so most folks just use "CBS" for short) plays a surprisingly quiet but critical role. CBS helps rubber cure at the perfect pace, making sure tires, industrial hoses, gaskets, and belts end up with the resilience they need. Anyone who’s tried fixing a bike tire in summer knows how temperature swings mess with rubber—chemistry behind vulcanization can make or break a product’s durability. Without CBS, the manufacturing lines for tire giants like Michelin, Goodyear, or smaller local producers would look a lot different—and not in a good way.
For heavy trucks, passenger cars, aircraft, and even scooters, the rubber compound recipe counts on this accelerator for strong, flexible, long-lasting results. Millions of vehicles depend on this chemical to keep rolling smoothly because it balances speed of production and quality in vulcanization, directly influencing road safety and cost for consumers and manufacturers alike.
In shoe factories worldwide, CBS steps up again. Sneakers, boots, sandals—each pair relies on soles that hold up to wear, weather, and pounding sidewalks. Athletic shoes, in particular, wouldn’t be the same without the spring and traction made possible by sturdy rubber blends. Big sports brands keep close tabs on the curing process that CBS supports since consumer loyalties ride on how shoes perform under daily stress. People expect soles not to split, crack, or lose shape, and good chemistry makes the difference.
Anyone tackling construction understands the perks of reliable sealing and insulation. Rubberized coatings, expansion joints on bridges, window claddings, and waterproof membranes in roofs draw strength and flexibility from chemical accelerators in their rubber content. CBS ensures that these products withstand temperature extremes, constant vibration, and years of exposure to the elements. Failure here leads to leaky buildings or weakened concrete structures—lost money and added headaches all around.
Think of all the sports gear, medical tools, and kitchen gadgets made from synthetic rubber. From stethoscope tubing to dishwashing gloves, these items face constant stretching, squeezing, and bending. Using CBS in the mix helps them last longer. Healthcare workers and home cooks alike depend on gear that doesn’t suddenly fail after a few uses. Safe, stable materials score higher in all regulatory checks, so the right accelerator selection protects both reputation and consumers.
Experience in manufacturing suggests that stricter environmental rules and worker safety concerns pop up whenever chemicals are at play. CBS carries some risk—like many industrial accelerators—so factories have to follow best handling practices, pay attention to protective equipment, and partner with suppliers who can provide testing data and support. Europe’s push for “greener” accelerators opened the door for ongoing innovation, nudging the whole industry toward safer production and lower emissions. Factories keep their certifications, workers stay healthy, and end-users get products they can count on.
Finding solutions that balance performance, cost, and safety means continuous research. Biobased or lower-toxicity accelerators may play a bigger role in the future, but for now, CBS holds its ground in core manufacturing sectors. Manufacturers and regulators both watch the data, making sure critical components like CBS continue serving real needs without creating new problems.
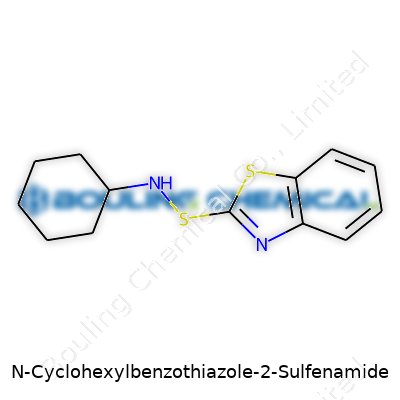
| Names | |
| Preferred IUPAC name | N-cyclohexyl-1,3-benzothiazole-2-carbothioamide |
| Other names |
CBS Santocure-CBS Vulcacit CBS N-Cyclohexyl-2-benzothiazolesulfenamide N-Cyclohexylbenzothiazole sulfenamide |
| Pronunciation | /ɛn-saɪkloʊˈhɛksɪlˌbɛnzoʊˈθaɪəzoʊl-tuː-sʌlˈfɛnəˌmaɪd/ |
| Identifiers | |
| CAS Number | 95-33-0 |
| Beilstein Reference | 358755 |
| ChEBI | CHEBI:35068 |
| ChEMBL | CHEMBL156210 |
| ChemSpider | 207427 |
| DrugBank | DB11474 |
| ECHA InfoCard | 100.051.755 |
| EC Number | EC 238-114-3 |
| Gmelin Reference | 82159 |
| KEGG | C14136 |
| MeSH | D002562 |
| PubChem CID | 160937 |
| RTECS number | DL3325000 |
| UNII | 1XVA01X37T |
| UN number | UN3077 |
| Properties | |
| Chemical formula | C13H17NS2 |
| Molar mass | 275.42 g/mol |
| Appearance | Light yellow crystalline powder |
| Odor | odorless |
| Density | 1.19 g/cm³ |
| Solubility in water | Insoluble |
| log P | 2.98 |
| Acidity (pKa) | 7.68 |
| Basicity (pKb) | 10.2 |
| Magnetic susceptibility (χ) | -0.58 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.647 |
| Viscosity | Viscosity: 2.1 mPa·s (at 20°C) |
| Dipole moment | 4.65 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 510.06 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -71 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -7708.8 kJ/mol |
| Pharmacology | |
| ATC code | D11AX10 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS05,GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | H302, H315, H317, H319, H335 |
| Precautionary statements | P261, P273, P280, P305+P351+P338, P337+P313, P302+P352 |
| NFPA 704 (fire diamond) | 1-2-0- مز |
| Flash point | > 127°C |
| Lethal dose or concentration | LD₅₀ oral rat 3,120 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral-rat LD50: 5100 mg/kg |
| NIOSH | NA; not listed |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for N-Cyclohexylbenzothiazole-2-Sulfenamide: "Not established |
| REL (Recommended) | 6 mg/m³ |
| Related compounds | |
| Related compounds |
2-Mercaptobenzothiazole N-tert-Butylbenzothiazole-2-sulfenamide N-Cyclohexyl-2-benzothiazolesulfenamide N-Oxydiethylene-2-benzothiazolesulfenamide N-Benzyl-2-benzothiazolesulfenamide |