Chemistry keeps evolving, and N-Chlorosuccinimide (NCS) is a reminder of that journey. Decades ago, organic chemists needed a way to introduce chlorine atoms into molecules without using hazardous chlorine gas. NCS stepped up as a reliable solid reagent and found a place in labs everywhere. Early work pointed out just how practical this compound could be for chlorination reactions. Its trajectory has followed advances in organic synthesis, shifting from specialty shops into the toolkit of academic and industrial chemists alike. I remember old chemical catalogs from the 1970s highlighting NCS as a safer, more predictable option for chlorination work, which always seemed to fascinate students first learning about reagents.
NCS usually appears as a white to off-white crystalline powder, packing enough building-block power to influence hundreds of reactions. It stands out in the long list of N-haloimides. Whether stored on glass shelves or kept in temperature-controlled rooms, people count on it for its high purity. Labs that need to run clean, reproducible reactions keep bottles of NCS handy, and seasoned chemists know their preferred brand. Commercial suppliers maintain tight quality controls, aiming to keep contamination or decomposition from spoiling reactions.
Looking at the numbers, NCS holds a molecular weight of 133.56 g/mol, and fits well in regular organic solvent systems. Its melting point hovers near 150°C, but those crystals begin releasing active chlorine even under milder conditions. Solubility in polar solvents like acetone and DMF comes in handy during chlorination, while water keeps it mostly undissolved. One sniff reminds users of its chemical potency, hinting at the reactive chlorine attached to its structure. The crystalline texture offers easy handling, but the stuff can degrade slowly in moist air. Keeping the container tightly sealed goes beyond simple caution—this chemical’s reactivity deserves respect.
Suppliers always stress purity—analytical grades often reach 98% or higher, and impurities like succinimide or dimeric side products stay well below threshold limits. Labels specify chlorination capacity and warn about safe storage temperatures. In the lab, stability checks matter, and thorough documentation lets users match chemical lots for reproducible results. Shipping labels reinforce regulatory compliance, since NCS needs careful handling on the road as well as on the bench. Storage away from direct sunlight and heat becomes second nature to those with experience.
Manufacturing keeps things straightforward. An aqueous solution of sodium hypochlorite brings about the chlorination of succinimide. Sodium hypochlorite, basically household bleach, streams into a cooled and stirred batch of succinimide. The product crystallizes out, washed and dried, then sieved for consistency. Some plants adopt flow chemistry routes to cut down on waste or streamline isolation. Lay chemists pick up on the wisdom here—avoid over-chlorination, keep the batch cold, and don’t breathe the fumes. Even as synthetic routes modernize, the classic batch recipe sets the standard for most commercial suppliers.
Few molecules have shown up in as many undergraduate labs as NCS. Alkene chlorination often leads the list, where NCS delivers just one chlorine atom, usually with high selectivity. Aromatic substitution reactions also lean on this reagent. Free radicals form easily thanks to the active nitrogen-chlorine bond, so NCS works as a gentle, controlled chlorinating agent in reactions where more aggressive chemicals would ruin the product. Some synthetic chemists tweak the N-haloimide structure itself, swapping out succinimide for phthalimide or other skeletons to fine-tune reactivity. The versatility of NCS means that dozens of published protocols cite it for halogenation, often adjusting temperature or solvent based on the substrate. The convenience of this reagent has helped push medicinal chemistry forward, especially as new heterocycles roll off the bench and into drug discovery pipelines.
Plenty of names tag along with N-Chlorosuccinimide, giving chemists a bit of confusion the first time through a catalog. NCS tops the list as the shorthand, but commercial packaging might read N-Chlorsuccinimid, N-Chlorbernsteinsäureimid, or just chlorsuccinimide. Each name points at the same white crystalline solid, so veterans learn to check CAS numbers (128-09-6) to stay clear about what sits in the bottle. In some markets, proprietary blends come with company codes, though the main component stays the same. Some textbooks refer to "chlorosuccinimide" without the "N," which can lead to confusion unless the context is clear.
Working with NCS demands respect, not just basic PPE. The stuff can irritate skin and eyes, and dust can trigger sneezing or worse if inhaled. Splash goggles and chemical-resistant gloves make a big difference. Inhaling dust or ingesting the solid means immediate trouble, and the local safety data sheet spells out what emergency measures to take. In my own work, I keep a chemical spill kit at hand, as NCS can cause hazardous chemical burns. Fume hoods stay running whenever this chemical comes out, and all waste tracks into designated containers—never pour leftovers down the drain. Facility managers keep training updated, knowing that accidents rarely forgive shortcuts. Reaction cleanups get just as much attention; even small residues can corrode or degrade if ignored.
Pharmaceutical chemists use NCS to introduce chlorine atoms into drug candidates, seeking better metabolic stability or sharper biological activity. Fine chemicals manufacturers run large batches for pesticide intermediates, dyes, and performance materials. Research groups value NCS for regioselective halogenation and controlled oxidation of organic molecules. The reagent even surfaces in the electronics industry, where specific chlorinated intermediates need controlled production routes. Scale varies—a few hundred milligrams for a bench-top synthesis, up to multi-kilogram lots for pilot plants. In each case, NCS bridges academic innovation and commercial production. I have seen early-stage drug projects succeed because they could make unique chlorinated scaffolds using nothing more exotic than NCS in a cold flask.
R&D teams keep probing for greener, safer, and more selective versions of NCS reactions. Automated synthesis robots can run dozens of NCS-based reactions in parallel, translating discovery into faster screening of potential products. Modern analytical methods—NMR, LC-MS, IR—make it easier to verify purity and detect side products, boosting the odds for better reproducibility. Researchers pivot toward lower-waste chlorination, swapping out solvents or tweaking reagent ratios. Some groups push for NCS analogs that offer specific reactivity, helping avoid the unwanted byproducts that slow down scale-up. Academics present fresh papers each year on new variations, which trickle down to commercial labs as teams hunt for more sustainable pathways.
Toxicologists underline the need for good controls with NCS, stressing known irritant properties and potential for byproduct release in high-volume settings. Chronic exposure, even at low levels, builds up over time and can sensitize workers, making strong ventilation and monitoring essential. Studies highlight that accidental ingestion causes gastrointestinal distress, while inhalation dries and irritates mucous membranes. Animal tests have mapped out acute exposure symptoms, helping update workplace standards. I have met colleagues who once shrugged at glove use, only to struggle with skin rashes after several weeks of exposure. Companies push for regular hazard assessments, keeping incident rates low with training and vigilance.
NCS continues to anchor classic synthetic routes, but fresh technology invites even bigger roles. Flow chemistry promises safer, high-throughput halogenations with less waste, making NCS even more attractive for scaling up fine chemicals. Custom modifications, where researchers adjust the succinimide ring or switch out the halogen, suggest new reagents just around the corner. Regulatory shifts mean that manufacturers must tighten documentation, traceability, and environmental controls. Digital inventory and automation make storage and ordering less of a headache, streamlining workflow and reducing accidental waste. Academic teams keep looking for gentler, yet just as powerful, alternatives, but current demand shows that NCS will keep its place on storeroom shelves for years to come.
N-Chlorosuccinimide, often known as NCS, holds a steady presence in many chemistry labs. Its biggest appeal sits in its predictability. Chemists, including myself, rely on NCS to add chlorine to specific spots on a molecule, a process known as chlorination. This kind of selectivity matters in research—if you pick the wrong reagent, you end up with a shelf full of useless byproducts. NCS plays it straight, behaving in the way textbooks promise, which means less waste and faster discovery cycles.
Making or tweaking chemicals rarely feels glamorous from the outside, but chlorination drives much of what happens in crop protection, drug development, and material science. For example, pharmaceutical researchers often tweak one atom at a time to find more effective drugs. NCS turns up again and again as a go-to for fine-tuning the structure of molecules. Its clean reactivity keeps the process manageable. I’ve seen projects stall for weeks because an alternative reagent gummed up the works with impurities, racking up wasted effort and cost. NCS, in many cases, would have saved days.
Chlorine itself is a bear to handle—dangerous and tricky to control. Compared to working straight with chlorine gas, NCS comes safer in solid form and with a more measured hand. It delivers chlorine where it’s needed without spreading it across every available carbon in the molecule. This is critical in environments like API synthesis, where a single slip can send an entire batch off the rails. Many labs, including places I’ve worked, choose NCS specifically to avoid bulkier side reactions and unnecessary clean-up steps. Lab safety officers appreciate the reduced volatility too.
Green chemistry stands on everyone’s radar these days. NCS makes it a bit easier to cut back on corrosive side-products and wasteful solvents. Nobody wants to dump gallons of hazardous waste if a cleaner route is available. Using NCS isn’t a cure-all—it still requires proper handling and disposal—but it usually leaves less residue compared to wilder reagents. In one case, swapping in NCS allowed a team I worked with to switch to water-based solvents, shrinking both waste and overhead. Regulatory compliance gets simpler and costs drop when you’re not battling a mountain of hazardous leftovers.
NCS serves well, but it’s not perfect. It has its own set of hazards and storage quirks. Keeping it dry and cool staves off decomposition, but not all chemical users enjoy ideal storage conditions. Mishandling—like letting the compound touch skin or linger in open air—carries real risks. Training and solid lab routines help but won’t fix everything. Some groups are experimenting with alternative chlorinating agents derived from renewable sources, hoping to lessen both environmental and health risks even further. This shift could lead to wide industry changes if new solutions prove affordable and scalable.
For anyone who’s ever spent time behind a bench, NCS ranks as a trustworthy option. It brings value not because it’s flashy, but because it performs predictably and safely in most hands. Smart chemical choices—especially those that cut messes and keep workers safer—affect everything from timelines to the planet’s health. Keeping tabs on emerging green alternatives and investing in training will keep the industry moving forward, and may one day dethrone even reliable old NCS. Until then, it remains a foundation for many labs’ best work.
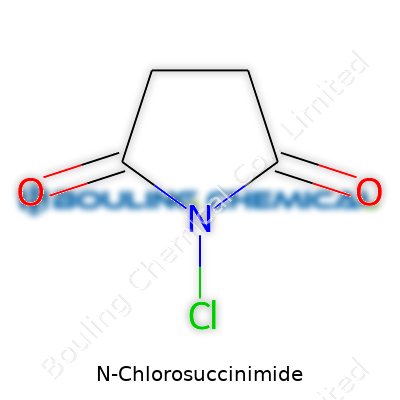
N-Chlorosuccinimide, simply written as C4H4ClNO2, crops up regularly in organic labs. In essence, this chemical combines the succinimide ring with a chlorine atom tucked away in the nitrogen position. Beyond its neat, compact structure, it represents a common tool used to introduce a chlorine atom into a compound during synthesis. Anyone who has spent time working in organic synthesis, whether on drugs, dyes, or plastics, probably recognizes the characteristic acrid smell and chalky texture of N-Chlorosuccinimide.
Some folks might brush past the small details of chemical reagents, thinking they're only relevant for bench chemists. That’s rarely true. N-Chlorosuccinimide is both a subtle and precise agent. Its main role shows up in selective chlorination, helping chemists direct a chlorine atom onto an aromatic ring or other molecular sites with remarkable accuracy. Chlorinated molecules form the basis for many common medicines, agrochemicals, and materials found in daily life. Without reagents like N-Chlorosuccinimide, creating those molecules could mean higher waste, lower selectivity, or even harsher reaction conditions.
Think about how much more effort and cost would come with relying on less-selective chlorination methods. For example, using gaseous chlorine brings clear health and safety risks—not to mention the challenge of keeping everything controlled and reproducible. N-Chlorosuccinimide offers a powder-based, manageable alternative, which matters to smaller laboratories or those working without extensive safety infrastructure. My own experience in student labs showed how using it almost felt like having a safeguard: safer, easier to store, less chance of an accident turning a simple procedure into a disaster.
Thousands of peer-reviewed articles highlight the efficiency of N-Chlorosuccinimide. Studies show it shortens reaction times, boosts product yields, and keeps by-products to a minimum. Beyond that, its application in medicinal chemistry has supported the discovery of antiviral and anti-inflammatory drugs. A 2022 review in the Journal of Organic Process Research summarized over 200 widely used reactions relying on this compound. Researchers favor N-Chlorosuccinimide for its substrate tolerance and the way it consistently delivers high yields, even under mild temperature and pressure.
Like any good thing, misuse or careless handling can pose risks. Exposure causes irritation and can trigger harmful fumes when mixed with acids. Labs regularly train chemists in best practices: sealed gloves, decent ventilation, prompt cleanup, and proper disposal. Thinking about the environmental load, alternatives and greener chlorination strategies have gained steam. Some researchers turn toward electrochemical or bio-inspired chlorination. These novel routes reduce dependence on substances like N-Chlorosuccinimide, but the chemical remains steady as a reliable go-to for challenging transformations. Regulatory agencies now also keep a close eye on workplace usage, waste management, and new methods.
Using N-Chlorosuccinimide often means striking a balance between practical convenience and environmental care. Lab supply chains have improved transparency, offering better information about sourcing and purity levels. Knowledge—not just the chemical formula—counts most in the drive for sustainable science. Real progress means every chemist, from undergrad to industry veteran, stays aware of both benefits and tradeoffs of tools like C4H4ClNO2, valuing safety and responsibility as highly as efficiency.
I worked for years handling and cataloging chemicals in an academic research lab. N-Chlorosuccinimide, known in the field for its usefulness in organic synthesis, demands respect in storage practice. Over the years, I’ve seen what happens if you ignore the fine print with chemicals like this. Problems range from loss of reagent strength to actual safety risks, costing both time and money.
Keeping N-Chlorosuccinimide dry matters. It reacts with water, even the humidity in air, degrading into less useful byproducts and sometimes releasing problematic vapors. I learned quickly that a simple screw-cap bottle on a regular shelf wasn’t enough. The compound often gets clumpy and yellow after a few weeks in a humid space, making accuracy in experiments impossible.
A desiccator changed everything. Silica gel or other active desiccants draw away what moisture might sneak in during quick openings. Some labs use nitrogen or argon to flush out air, especially in climates where the humidity never dips. My own solution: small, tightly sealed glass bottles inside a dry, dedicated chemical cabinet away from water and direct air currents. That layer of thoughtful storage has kept my NCS stock fresh long past the expected shelf life.
Heat speeds up the breakdown of N-Chlorosuccinimide. Room temperature is safe if you avoid the furnace room, window ledges, and the top shelf above radiators. I found that in the summer, temperature spikes in storerooms put the chemical at risk, so I once moved my supply to a cool, ground-floor cabinet. Even a few unplanned days of excessive warmth will shorten the chemical’s viability. Some companies recommend refrigeration for bulk supplies, but condensation becomes a risk, so always let the bottle reach room temperature before opening.
N-Chlorosuccinimide breaks down with exposure to sunlight and strong laboratory lights. Nobody wants to find a pale yellow powder transformed into a brown, useless mess. I switched to amber glass containers after one ruined batch, leaving the bottle in the back of a cabinet where any daylight can’t reach it. Blackout storage containers work even better for labs in bright, sunlit spaces.
Mixing the wrong chemicals in one cabinet spells disaster. N-Chlorosuccinimide gives off chlorine gas with strong acids or bases, especially if a bottle leaks or cracks. Separation isn’t just product safety — it’s personal safety. My own system followed OSHA hazard class segregation signs: oxidizers in one section, acids another, bases another, and so forth. A clear inventory keeps things simple, and accidental mixing never caught me by surprise.
Even great storage turns bad with poor labeling. Date your N-Chlorosuccinimide bottle as soon as it’s delivered. Keep lot numbers and expiration information visible. I checked seals and color every time I reached for the bottle, pulling any suspicious-looking batch for review. If a powder looked off or formed clumps, I disposed of it in the designated chemical waste area — never down the drain.
Safe, effective chemical storage brings peace of mind and prevents accidents. Day-to-day diligence, not shortcuts or wishful thinking, keeps dangerous surprises at bay. Check your bottles, keep containers dry and cool, block out the light, and give every chemical the respect it asks for. Experience proves it’s worth the effort.
I’ve worked with a lot of chemicals in my time as a lab technician, and N-Chlorosuccinimide, also known as NCS, always demanded respect. This chemical plays a big role in organic synthesis, helping chemists prepare everything from medicines to dyes. But beneath its usefulness, NCS comes with a set of health hazards that shouldn’t fade into the background.
NCS structures look pretty mild on paper, but its reactivity gives it quite a punch in the lab. On skin, it can cause immediate irritation, sometimes redness, even burns. Eyes are a bigger worry: a single splash brings stinging pain, and can lead to damage that takes weeks to heal. During my own early years in the laboratory, a colleague dropped some on his glove and within moments that latex started showing wear. That image has stuck with me.
Inhalation poses another risk that’s not easily dismissed. NCS can release chlorine-like fumes that bring on coughing or chest tightness. Chronic exposure raises the stakes, irritating the nose, throat, and potentially causing long-term respiratory problems. There’s no dramatic warning, just a slow increase in discomfort that’s easy to ignore until it adds up.
The Agency for Toxic Substances and Disease Registry labels chlorinated compounds—NCS included—as hazardous unless handled inside a working fume hood. Look up any material safety data sheet (MSDS): eye, skin, and lung hazards come up every time. The American Conference of Governmental Industrial Hygienists recommends only trace level exposure over a work shift.
Accidents don’t just live on paperwork. At a pharma plant where I consulted, an NCS spill forced an evacuation. Two engineers forgot their goggles. Both landed in the emergency room for eye flushing. The company overhauled its PPE policies after that incident. These are the real stories that add grit to the numbers.
Gloves, goggles, and a fume hood are baseline protection when NCS is around. They’re not just boxes to check off; they block contact and keep lungs clear. I noticed the best labs invest in proper ventilation and run routine safety drills. That builds a healthy respect for what these chemicals can do, and keeps emergencies from spiraling.
Training newcomers is where I’ve seen the most room for improvement. Book knowledge doesn’t stick until someone sees a demonstration or watches a “fail” the safe way. I make a point to show the effect of NCS on a nitrile glove. Seeing the glove degrade brings home the message more than any slide deck. That kind of practical awareness can’t be replaced.
Beyond equipment and training, it’s worth looking for alternatives. Green chemistry keeps making progress, offering less hazardous options for several reactions that historically needed NCS. Switching can cut down the daily risks and waste at the same time.
Strong policies that go beyond the rulebook give everyone a clearer sense of what’s at stake. Safe storage, clear labeling, and planned routes for disposal matter more than their spot on a checklist. Leadership that listens to lab workers—asking about near-misses, reviewing close calls—builds a culture where fewer people get hurt. We all play a role in shaping that culture, and every safe shift is proof that it makes a difference.
Anyone who’s spent time in a lab knows that running reactions feels a lot like cooking—except the ingredients come with longer names and stricter recipes. If you’re working with N-Chlorosuccinimide, or NCS, chances are you’re looking to introduce chlorine into an organic molecule with some precision. Getting the molar mass right is critical here. Every measurement matters, and a single misstep can throw the results off, trigger an unwanted side reaction, or even pose safety risks. In all my years of weighing out chemicals, attention to this number has always served as a pillar of good practice.
The formula for N-Chlorosuccinimide is C4H4ClNO2. To figure out the molar mass, you need to look at what’s under the hood. The atomic weights sit roughly as follows: carbon at 12.01, hydrogen at 1.01, chlorine at 35.45, nitrogen at 14.01, and oxygen at 16.00. Doing the math for a single molecule gives us:
If you add these values together, the molar mass comes out to 48.04 + 4.04 + 35.45 + 14.01 + 32.00, giving a sum of 133.54 grams per mole. That number travels with the bottle and sits on every datasheet, but it goes far beyond just number crunching; it forms the foundation for every calculation that makes research move forward safely and smoothly.
The right molar mass ensures stoichiometry stays on point. Overshooting leads to waste and sometimes toxic byproducts. Underestimating can stall a reaction or limit its yield. Both situations drain resources, eat time, and impact results. Having seen failed syntheses up close, the lesson hits home quickly—know your reagents well, starting with the basics.
With N-Chlorosuccinimide, weighing by molar mass keeps things predictable. Analytical chemists and bench scientists rely on that reliability whether they’re halogenating a substrate or running a large-scale process. In my own work, mistakes in measurement have been rare but unforgettable, leaving no doubt about the importance of double-checking the chemistry upfront.
Careful sourcing of chemicals helps catch small discrepancies. Some suppliers provide purity ratings or batch-specific certificates of analysis. Trusted sources, solid documentation, and well-calibrated balances become part of the solution, helping prevent the kinds of setbacks that come from incorrect weights.
In teaching students, I encourage not just memorization of values, but understanding the process of how those values come together. This approach helps avoid simple slips—like reading the wrong line on a periodic table—or larger mishaps, such as assuming one batch matches another. Emphasizing these checks forms a habit that consistently pays off, whether scaling up a synthesis or confirming the purity of a product.
Getting technical details right, including finding and applying the correct molar mass for compounds like N-Chlorosuccinimide, stands as a core aspect of chemical research and industry. With this approach, chemists build trusted data, ensure safe practices, and deliver accurate results that keep discovery moving forward.
| Names | |
| Preferred IUPAC name | 1-chloro-2,5-dioxopyrrolidine-1-carboxamide |
| Other names |
NCS 1-Chlorosuccinimide Succinimide, N-chloro- N-Chlorsuccinimid N-Chlorsuccinimide |
| Pronunciation | /ɛn-klɔːroʊ-səkˈsɪnɪmaɪd/ |
| Identifiers | |
| CAS Number | 128-09-6 |
| Beilstein Reference | 120793 |
| ChEBI | CHEBI:48041 |
| ChEMBL | CHEMBL1418 |
| ChemSpider | 5606 |
| DrugBank | DB11462 |
| ECHA InfoCard | 03b981e7-6faf-4e87-81e7-68c04e8a019a |
| EC Number | 3.1.1.3 |
| Gmelin Reference | 82286 |
| KEGG | C06715 |
| MeSH | D002792 |
| PubChem CID | 8556 |
| RTECS number | WN3325000 |
| UNII | Q7AVC2V78K |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DTXSID0034262 |
| Properties | |
| Chemical formula | C4H4ClNO2 |
| Molar mass | 133.56 g/mol |
| Appearance | White to off-white crystalline powder |
| Odor | Chlorine-like |
| Density | 1.4 g/cm³ |
| Solubility in water | slightly soluble |
| log P | -0.96 |
| Vapor pressure | <0.01 mmHg (25°C) |
| Acidity (pKa) | 6.0 |
| Basicity (pKb) | 7.55 |
| Magnetic susceptibility (χ) | -6.9 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.499 |
| Viscosity | 1.02 mPa·s (at 20 °C) |
| Dipole moment | 3.41 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 311.6 J·mol⁻¹·K⁻¹ |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and eye irritation, may cause respiratory irritation, oxidizing agent. |
| GHS labelling | GHS02, GHS07, GHS09 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P273, P280, P301+P312, P305+P351+P338, P304+P340, P330, P337+P313, P501 |
| NFPA 704 (fire diamond) | 2-0-1-OX |
| Autoignition temperature | 335 °C |
| Lethal dose or concentration | LD₅₀ (oral, rat): 1500 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50: 1800 mg/kg |
| NIOSH | SN2980000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.1 mg/m³ |
| Related compounds | |
| Related compounds |
N-Bromosuccinimide N-Iodosuccinimide Succinimide |