N-Butylpyrrolidine entered the chemical scene way before anyone started paying close attention to the full lineup of substituted pyrrolidines. Tracking its roots takes us back to the post-war years when organic chemists, spurred on by necessity, looked to build new solvent options and intermediates for drug synthesis. Its story ties in with the growing use of pyrrolidine structures in everything from pharmaceuticals to agrochemicals. As researchers figured out its value as a flexible building block, especially in asymmetric synthesis and alkaloid analog production, it grew into a staple for any lab working with nitrogen-containing rings. The tried-and-tested nature of its synthesis—often a simple alkylation with the parent pyrrolidine—made it accessible in bulk, shaping its broad presence across multiple industries.
N-Butylpyrrolidine isn’t a showstopper visually or in terms of odor, but beneath that unobtrusive exterior, it serves an essential purpose. Chemists see it as a four-carbon ring with a butyl tail sticking off the nitrogen, giving just enough of a hydrophobic character to alter the parent compound’s solubility and reactivity. Its main strengths come out in its role as an intermediate for more complex molecules. Though primarily a laboratory commodity, supplier sheets often mention shipment as a colorless liquid in metal or polymer containers, clearly marked with batch and hazard labels. It often rides on logistics supply lines alongside other pyrrolidine derivatives as pharma and agrochemical plants churn through vast liters of these building blocks every season.
N-Butylpyrrolidine’s liquid state at room temperature makes it easy to handle on a manufacturing or lab scale. Its boiling point hovers around 175–180°C and the melting point keeps well below freezing, so cold storage doesn’t cause issues. The density sits just shy of water, easing separation in biphasic reactions. Moisture doesn’t play nice: water-uptake diminishes stability, so keeping it capped and somewhere dry saves headaches. Chemically, the butyl group cuts the basicity seen in its parent, making this compound a bit less punchy than straight pyrrolidine. The ring’s moderate nucleophilicity lets it stand up in SN2-type reactions or participate in constructing bigger, more elaborate molecules. The compound dissolves well in most non-polar solvents and is often a friend to those wanting to shift reactivity using alkyl chain modifications.
Suppliers highlight key numbers—purity typically above 98%, water content kept to a minimum, and a clear report of heavy metal content to meet pharmaceutical precursor standards. Labels jump out with a GHS warning sign due to volatility and potential health risks. On the SDS sheet sits a clear rundown of storage, handling, emergency guidelines, plus the usual hazard pictograms. CAS number 3470-98-2 and synonyms signal this is N-butylpyrrolidine, with UN shipping numbers flagged for logistics teams. In practice, all this labeling isn’t window-dressing. Any slip in documentation could trigger regulatory trouble or safety incidents, so everyone from warehouse staff to bench chemists keeps a close eye on what’s coming in and out of storerooms.
Most production starts with pyrrolidine reacting with a butyl halide—usually butyl bromide or chloride—in the presence of a base such as potassium carbonate. This classic nucleophilic alkylation spins up on industrial reactors or even hotplates in teaching labs. Going for higher yields, technicians dry solvents carefully and monitor temperature, since side-products and low-boiling residues can cause loss or produce impurities. Chasing after higher throughput and cleaner reactions, some plants run the process in continuous flow mode rather than classic batch reactors. The intermediate byproducts—salts and unreacted starting material—often recycle back into the process, reducing waste and cutting costs.
The butyl tail offers interesting opportunities at the bench. It dampens basicity of the nitrogen compared to straight pyrrolidine, which can prove handy or limiting depending on target molecules. Researchers regularly lean on reductive or oxidative transformations on the ring itself, and chemoselective acylation at the nitrogen opens doors for peptide or alkaloid analog synthesis. One lab I spent time in looked at N-alkylated pyrrolidines as starting points for chiral ligands, tweaking enantioselectivity in transition metal catalyzed reactions. Stripping or swapping the butyl group isn’t straightforward because secondary amines can overreact, but in the hands of an experienced synthetic chemist, N-butylpyrrolidine keeps surprising with its adaptability.
Depending on where you source it, expect to see names like 1-butylpyrrolidine, N-butyl-tetrahydropyrrole, or just plain N-butylpyrrolidine. Catalogues in bulk chemical houses, especially across Europe and East Asia, often use the systematic or IUPAC names to avoid any confusion. Harmonized names help with customs and import checks, speeding up orders in the maze of international logistics. Whichever moniker it wears, savvy buyers double-check CAS numbers to keep mistakes off the production floor.
N-Butylpyrrolidine brings some hazards to the table. Volatility creates an inhalation risk in open systems, and skin contact leads to irritation. Most production and handling settings lean hard on fume hoods, splash-proof goggles, and gloves. For fire safety, the compound’s flash point—about 61°C—means sources of ignition need careful control. Fire department spreadsheets categorize it under the same risk class as many organic amines, demanding chemical extinguishers on deck rather than water jets. In small-scale research, I always booked time in specialty labs with full spill kits, and strict waste management tracked where every drop ended up. Regulations in most countries now ask for regular risk assessments, with data sheets and audits part of daily routine for any company importing or making this compound.
In the pharma world, N-butylpyrrolidine often finds itself as a stepping stone toward complex nitrogenous drugs. Some antihistamines and analgesics get built from this structure, using it to tune solubility or metabolic resistance. Beyond drugs, agrochemical synthesis uses it to build up pesticide and fungicide scaffolds, especially those that draw value from alkylated amines. As a solvent or additive in electronics manufacturing, the compound’s usefulness pops up in cleaning processes that need something stronger than simple alcohols but less reactive than the full suite of amines. Over the past few years, research teams have explored polymer functionalization using N-alkylpyrrolidines, opening new windows for advanced materials and adhesives.
Recent literature features plenty of case studies for asymmetric synthesis involving chiral N-butylpyrrolidine derivatives. Process chemists keep tinkering with reaction efficiency, solvent choices, and greener synthetic protocols to drive down environmental impact and energy use. Collaboration between industry and academic labs still pushes the boundaries, weighing up cost versus performance as new applications crop up. Some grant-funded projects look at computational approaches to mapping reactivity, hoping to replace guesswork with solid predictions for selectivity and scalability. In my own experience, joint teams sometimes get competitive, racing to file patents for new routes or product claims before rivals publish or go public with pilot-scale runs.
Sifting through animal studies and toxicology profiles, results show moderate acute toxicity—an outcome in line with many alkyl amines but not in the “direct hazard” bracket. Inhalation and ingestion both cause discomfort at relatively low exposures, with long-term effects on organ systems still under review. Most research focuses on short-term contact and occupational hazards, so chronic studies and epidemiological data lag behind. Any operation that stores or ships large quantities maintains strict reporting schedules for spills, exposures, or process upsets. As with countless other organic reagents, safe handling protocols develop as new data emerges and regulators update permissible exposure limits.
As industry pivots toward green chemistry, N-butylpyrrolidine’s straightforward synthesis and ready availability might make it a more popular substitute for some less efficient amine reagents. Research into biodegradable derivatives, especially those with lower toxicity and easier waste treatment, shows some promise over the next decade. Advances in catalysis and process design could cut down on energy use and side-product formation, boosting competitiveness in a crowded chemicals marketplace. Looking ahead, there’s every reason to expect that labs and manufacturers will keep finding new purposes for this modest-looking building block, particularly as pressure mounts to source raw materials more responsibly and cut down on hazardous waste streams.
Most people never hear about N-Butylpyrrolidine outside of a lab. I stumbled across it once during a college chemistry project, thinking its name sounded more like a code than something that plays a supporting role in making everyday products. This molecule shows up as a building block in the process of creating certain drugs, flavors, and fragrances.
Big drug companies use it in organic synthesis, meaning they build more complex molecules out of simple ones. N-Butylpyrrolidine helps tweak a drug’s structure to improve how it works. One pharma company I visited stored it behind a double-locked door, right next to rows of glass vials for testing new painkillers. They use it because it can donate and accept various chemical groups, speeding up the chemical reactions that give us everything from antibiotics to asthma meds.
Apart from medicine, flavor houses look at amines like N-Butylpyrrolidine to punch up artificial seasonings. It helps blend flavors so the chips or candy taste sharper and more consistent. This isn’t the molecule you taste, but it’s a rung on the ladder that gets the job done. Some companies have said their quality control teams track every batch that uses a pyrrolidine derivative, since the right amount can make or break a flavor.
N-Butylpyrrolidine drops into fragrance production, too. The colognes you walk past at the mall sometimes owe their bite or smoothness to similar compounds. Chemists test small changes in its structure to get new scents that stand out against a wall of flowery or musky options. This means people turning their nose up at synthetic fragrances forget just how much trial and error happens with molecules you never hear about.
Some folks shrug at the mention of another specialty chemical. The thing is, these building blocks shape modern life. Without compounds like N-Butylpyrrolidine, making affordable medicine or new flavors would take longer, cost more, and sometimes be less effective. My own experience in research told me that even the most marginal chemical can ripple out to bigger changes in products we take for granted.
Another reason to pay attention is safety. N-Butylpyrrolidine carries risks that shouldn’t get swept under the rug. Spills in the lab can mean headaches or worse if it isn’t handled right. Some inhalation tests on rodents have raised health concerns, pushing companies to limit worker exposure and use better protective gear. Watching friends double-glove and work under vents gave me a new respect for chemical safety rules that some see as nitpicking.
One challenge with substances like N-Butylpyrrolidine is balancing their usefulness with their hazards. Cleaner production methods have been slowly replacing older, dirtier ones. The push for green chemistry means research teams are trying safer alternatives or figuring out ways to recycle solvents instead of dumping them. Companies in Europe have started publishing their toxicology reports to clue others in, opening the door for tighter regulation and better worker protection.
More transparency and stricter handling go a long way. I’ve seen labs where safety signs blend into the background, but after a close call with a leaky flask, my old team put protocols front and center—eye washes, extra filters, more frequent training. These steps don’t eliminate the risks, but they make accidents less likely and keep the focus on using N-Butylpyrrolidine safely, so new drugs or flavors can keep showing up on shelves without putting anyone in harm’s way.
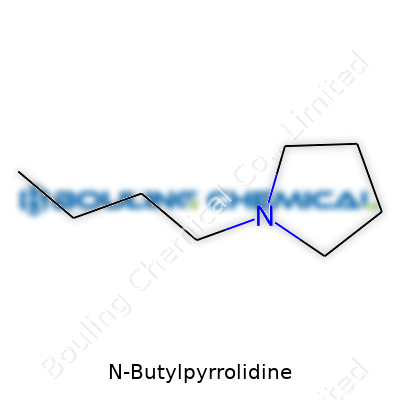
Chemistry often feels like a sea of intimidating names and formulas. You hear “N-Butylpyrrolidine,” and it sounds more like a password than a real substance. Strip away the jargon, and what you find is a molecule with a straightforward setup: a five-membered ring called pyrrolidine, hooked up to a four-carbon butyl chain off the nitrogen.
The molecular formula, C8H17N, breaks down like this: eight carbons, seventeen hydrogens, and a single nitrogen. The butyl group (C4H9) attaches directly to the nitrogen atom in the pyrrolidine ring, not hanging off by some indirect bond. It looks a bit like a hula hoop with a tail. And that tail, the butyl chain, tacks on a new set of properties.
Having spent years doodling chemicals on the backs of notepads, I've found rings like pyrrolidine pop up everywhere—solvents, drugs, funky materials. If you sketch N-Butylpyrrolidine, you get a tight, slightly puckered five-membered ring with four carbons and a nitrogen. Picture a pentagon where one corner is a nitrogen, and from that nitrogen a butyl chain stretches outward, almost wagging.
The way that chain joins to the nitrogen—direct, without extra carbon separation—changes how this molecule behaves. Adding that butyl chain makes the molecule less water-loving and more comfortable in oils or organic environments. This means it slips more easily into nonpolar chemicals, just as oil floats on top of water: opposites don’t always mix well.
I've sat in stuffy labs watching how small changes to a molecule tweak its entire personality. For N-Butylpyrrolidine, slotting a butyl on the pyrrolidine nitrogen alters how it interacts with the world. Other pyrrolidines feel sharp, almost biting when you open the bottle. Add a butyl, and things mellow out. The oiliness, the way it dissolves in organic solvents, even the boiling point, shift with the swap.
This isn’t just academic nitpicking. Tweaking structural elements guides chemists in building new drugs, designing better batteries, or coming up with fine-tuned solvents. Chemists rely on the details: the balance of carbon, hydrogen, nitrogen, and the position of each group. The blueprint, the formula, and the shape all work together, dictating how the molecule acts in the wild.
N-Butylpyrrolidine carves out space in specialty chemical synthesis. As labs chase greener methods, adjusting classic molecules like this can reduce unwanted byproducts or limit dangerous waste. Introducing a butyl group can even make recycling or disposal less risky, since its solubility profile changes. It’s not a fix-all, but every tweak offers tools for creative problem-solving.
Navigating chemistry’s maze taught me to respect these details. Each atom, each branch off a molecule’s core, matters. It’s tempting to skip straight to end results, but progress depends on knowing the nuts and bolts. N-Butylpyrrolidine, simple as it sounds, is one of those nuts and bolts. With tougher demands showing up in greener manufacturing and safer chemistry, small changes like this add up in surprising ways.
N-Butylpyrrolidine has a pretty specific character. Anyone who’s worked in a lab knows that certain chemicals demand respect, not just for their own sake, but for everyone’s safety. N-Butylpyrrolidine falls into that group. It doesn’t blow up in your face, but ignoring its requirements makes for messy, sometimes dangerous situations.
This chemical prefers a tucked-away spot. Think cool, well-ventilated rooms—no tropical heat, no damp cellars. I learned early on that even a short stretch above room temperature nudges the risk of unwanted reactions, especially if traces of moisture get involved. Any storage area should offer solid airflow so vapors don’t settle and hang around. I’ve watched folks mishandle solvents in a tight closet, and the headaches—from fumes alone—make you realize ventilation isn’t just a checklist item.
Containers count. N-Butylpyrrolidine happily chews through some plastics over time, so stainless steel or glass wins out every time. Anyone keeping this stuff long-term always checks that seal. No cracks, no leaks, no improvisation with mystery jars. A busted container once turned an ordinary afternoon into a full evacuation where I worked—an expensive, embarrassing lesson for the team.
Mislabeling creates confusion, especially when the chemical names all sound the same. I’ve seen temp workers accidentally pour N-Butylpyrrolidine into the wrong waste drum. That turned into a week of paperwork. Right labels, clear dates, and warnings about what’s inside keep everyone moving safely.
This chemical doesn’t deal well with strong oxidizers and acids. The reaction isn’t always dramatic, but it releases hazardous gases—nobody wants to breathe those in. Shelves and storage cabinets need real physical separation (think different shelves, even different cabinets) to avoid those classic mix-ups.
Gloves and goggles come out every single time, no excuses. Even though skin contact might not seem terrible right away, I’ve watched coworkers develop rashes and respiratory issues after getting careless. And no eating or drinking near this stuff. It sounds obvious, but people cut corners and pay for it with their health.
Spills do happen. I always tell new techs to mop up with inert materials (vermiculite, sand) instead of rags, and to skip the water—it can make spreading worse if things get out of hand. Fast containment beats frantic cleanup every time, so keeping spill kits nearby isn’t negotiable.
Waste disposal feels like a chore, but tossing N-Butylpyrrolidine down a sink or mixing it with regular trash is asking for trouble. Most places call in trained waste handlers. I still remember someone dumping leftover solvent straight into city drains and the wastewater plant calling our lab the next morning. It doesn’t just break the rules—it brings fines and puts people at risk.
Written protocols only do so much. Strong training for everyone handling chemicals builds good habits. I found that frequent walkthroughs—actually watching how people interact with storage and use kits—made more of an impact than a binder full of rules. Regular checks on ventilation, containers, and labeling mean problems get fixed before they turn into incidents.
Starting with the right space, equipment, and mindset makes all the difference. Respect for N-Butylpyrrolidine—the kind that comes from experience, not just a rulebook—keeps daily work smooth and safe.
Lots of folks don’t spend time thinking about chemicals like N-Butylpyrrolidine. I can’t blame them—who has the bandwidth, between juggling daily grind and family life? But conversations around these kinds of compounds do matter. They sneak into research labs, some industrial settings, and specialty manufacturing, often swept under the rug unless there’s an accident or health scare.
N-Butylpyrrolidine comes from a family of chemicals that’s not quite household stuff, but it’s handled in places where a slip-up can cause headaches—sometimes literally. Simple contact with skin or eyes can mean irritation almost right away, so lab coats and goggles stop being optional pretty fast. The hits don’t stop at the surface, either. Breathing in vapors, especially over time, invites coughing and burning sensations nobody wants to deal with at work.
One thing that always gets me: the way some of these substances creep by unnoticed until something goes wrong. You pour it into a beaker, a little fume wafts out, and the next thing you know, someone’s sneezing or worse. There aren’t any wild horror stories in the news linked to N-Butylpyrrolidine right now, but the absence of headlines doesn’t mean it’s safe to ignore basic precautions.
I think about how folks end up exposed. Maybe a cracked container, a fumble with gloves, or just carelessness during cleanup. Liquid chemicals seep through gaps, splash during transfers, or evaporate and hang in the air. Most workplaces with decent procedures keep those mishaps rare, but just about every veteran lab tech has a tale about someone cutting corners and regretting it.
Swallowing this stuff doesn’t sound likely, but stranger things have happened. Cross-contamination lurks everywhere unless everyone in the building scrubs up. And the risk doesn’t just end with human contact—improper disposal lets chemicals like this one slip into water supplies or soil. Those scenarios make me nervous and keep the environmental health folks on their toes.
Chemical manufacturers print out safety data sheets for a reason. In the sheet for N-Butylpyrrolidine, you’ll spot warnings about its flammable nature and advice for safe storage. It might not be as notorious as some solvents, but that flame symbol means business. The flashpoint hovers around 70°C, which is lower than you’d want for just leaving it out on a hot day in the wrong climate. Even seasoned chemists can forget and store things poorly, and fires ignite faster than most people expect.
In my own time working near chemical storerooms and research benches, I’ve seen simple steps save the day. Store chemicals in sturdy, labeled containers—never in old water bottles. Use fume hoods, seal lids tight, and post the Material Safety Data Sheet (MSDS) right where everyone can get to it. If a spill happens, trained folks tackle it right away versus panicking or ignoring the mess. Regular safety refreshers help new folks avoid repeating old mistakes.
Regulations keep some chemicals in check, but personal responsibility at the bench matters even more. If you’re working with N-Butylpyrrolidine, swapping stories and sharing best habits with colleagues pays off big time. Respect goes further than rules. Students and lab techs who build good habits early cut their risk, and that’s something every workplace should celebrate.
For a lot of people, N-Butylpyrrolidine sounds like something best left in a chemist’s notebook. In reality, this compound quietly supports industries that power daily life. I’ve spent time around manufacturing plants and watched how chemical choices affect everything from the end product to worker safety. Places you might not expect rely on ingredients like N-Butylpyrrolidine, each for their own reasons. Here’s how it plugs into a few major fields.
I’ve noticed how a little tweak in a drug recipe can mean the difference between relief and a recall. N-Butylpyrrolidine often helps when it’s time to build chemical structures that make up painkillers, antibiotics, or even drugs for chronic conditions. Its ability to act as a base in reactions lets manufacturers shape molecules precisely, improving the yield and sometimes purity of the final compound. Skipping corners isn’t an option here—safety standards keep everyone on their toes, so reliable intermediates keep products consistent batch to batch.
If you walk into a plant that produces fine chemicals, you’ll see long lists of raw materials coming in the back door. N-Butylpyrrolidine makes the cut for projects involving custom synthesis, where the job calls for unique chemicals that don’t sit on store shelves. As a secondary amine, it brings versatility to the table, helping assemble more complex molecules for everything from perfumes to crop protectants. While not every plant uses this compound, those that do value the way it slots into various reaction pathways without adding unwanted side effects to finished products.
Lab technicians, from undergraduate researchers to veterans in the field, appreciate when a solvent pulls its weight without causing headaches. N-Butylpyrrolidine offers a helpful hand in some extraction processes and chromatographic separations. Its solubility profile pairs well with organic materials, giving it a spot in the toolbox for analysts and process engineers. Across the corridor from the labs, scale-up teams favor dependable solvents for purifying products at production scale. Reliability counts—delays here ripple into bottlenecks fast.
Farmers rarely see the chemistry behind crop protection products, but their results show up plainly in the fields. N-Butylpyrrolidine’s utility in agrochemical synthesis means it plays a part in the formulation of herbicides and fungicides. The chemistry needs to be spot-on, or weeds and blight start winning. Working in agricultural labs taught me that small shifts in process ingredients can lead to big changes in product performance. Reliable sources of specific intermediates like this one keep essential formulations on the market and ensure seasons go smoothly for growers.
One thing I keep in mind: with every useful chemical comes a responsibility. Manufacturers who depend on specialty amines such as N-Butylpyrrolidine can face sourcing difficulties or regulatory scrutiny. Tightening rules around chemical handling call for greener production routes. Some companies invest in alternative synthesis methods with less environmental impact. Open communication between chemists, engineers, and regulators helps spot risks early and keep products flowing. Substitution isn’t always possible, but greater transparency and research can soften supply shocks and keep vital industries running.
| Names | |
| Preferred IUPAC name | N-butylpyrrolidine |
| Other names |
N-Butylpyrrolidine 1-Butylpyrrolidine Butylpyrrolidine |
| Pronunciation | /ɛn-ˈbjuːtɪl-pɪˈrɒlɪdiːn/ |
| Identifiers | |
| CAS Number | 3470-98-2 |
| 3D model (JSmol) | `/lcJ@H]WcGB@` |
| Beilstein Reference | 1109281 |
| ChEBI | CHEBI:131151 |
| ChEMBL | CHEMBL4225467 |
| ChemSpider | 135508 |
| DrugBank | DB16647 |
| ECHA InfoCard | 200-874-1 |
| EC Number | 211-002-2 |
| Gmelin Reference | 108026 |
| KEGG | C19139 |
| MeSH | D017174 |
| PubChem CID | 12572 |
| RTECS number | UJ4375000 |
| UNII | U05A4T1AP5 |
| UN number | UN2733 |
| CompTox Dashboard (EPA) | DTXCID90300128 |
| Properties | |
| Chemical formula | C8H17N |
| Molar mass | 141.26 g/mol |
| Appearance | Colorless liquid |
| Odor | Amine-like |
| Density | 0.824 g/mL at 25 °C |
| Solubility in water | miscible |
| log P | 0.96 |
| Vapor pressure | 0.437 mmHg (at 25 °C) |
| Acidity (pKa) | pKa = 11.27 |
| Basicity (pKb) | 2.84 |
| Magnetic susceptibility (χ) | -7.97 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.434 |
| Viscosity | 0.78 cP (20°C) |
| Dipole moment | 2.25 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 365.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -73.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4135.7 kJ/mol |
| Pharmacology | |
| ATC code | N05CM21 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Precautionary statements | Precautionary statements: P261, P264, P271, P280, P301+P312, P304+P340, P305+P351+P338, P312, P330, P337+P313, P405, P501 |
| NFPA 704 (fire diamond) | 1-3-0 |
| Flash point | 67 °C |
| Autoignition temperature | 310 °C |
| Explosive limits | Explosive limits: 1.1–8.0% |
| Lethal dose or concentration | LD50 (oral, rat): 500 mg/kg |
| LD50 (median dose) | LD50 (median dose): 300 mg/kg (oral, rat) |
| NIOSH | WA8060000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for N-Butylpyrrolidine: Not established |
| REL (Recommended) | 1 ppm |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
Pyrrolidine N-Methylpyrrolidine N-Ethylpyrrolidine N-Propylpyrrolidine N-Isopropylpyrrolidine |