Chemists in the early 20th century faced a problem: direct bromination reactions often proved unreliable, messy, or dangerous for precise laboratory work. N-Bromosuccinimide (NBS) emerged from this need, not as the result of a specific Eureka moment, but as the product of methodical tinkering in organic synthesis labs during the 1930s and 1940s. Rossiter and Evans are usually credited with isolating a practical form of NBS, giving researchers a safer and more predictable way to introduce bromine atoms into organic molecules. I remember as a student being struck by just how cleaner the NBS approach felt, compared to direct use of liquid bromine, a chemical with a terrifying reputation. Textbooks now highlight the move toward reagent control during these decades as a key shift in how organic chemistry experiments got done.
N-Bromosuccinimide stands out as a pale, chalky solid with a trick up its sleeve: it serves as a steady source of bromine in the lab, most often for bromination of allylic and benzylic positions but also for other nuanced organic transformations. Its selling point lies in its handling—NBS arrives in a jar as manageable crystals, not as a gas or a caustic liquid. Labs keep small glass bottles of it on hand, usually labeled with just the recognizable “NBS” shorthand and hazard pictograms. Chemists value the way NBS gives controlled amounts of bromine for selective reactions, which enables more finesse in product outcomes. Direct exposure to bromine gas or solutions can trigger panic even in seasoned chemists; NBS tames that risk to some extent.
This compound offers a melting point just above 170 degrees Celsius, although it decomposes before hitting a true liquid phase. In its pure form, NBS earns a reputation for stability under dry conditions, but introduce it to light, moisture, or heat, and reactivity ramps up quickly. Water solubility stays low, but organic solvents like carbon tetrachloride or acetonitrile work much better, making it a natural fit for organic labs. I’ve seen jars of it turn yellowish on the shelf when exposed to light, a warning sign that degradation and free bromine are sneaking in. The compound has a faint bleach-like smell that clings to gloves and glassware after use.
Labels for NBS typically flag its molecular formula—C4H4BrNO2—and offer a purity range, usually north of 98%, suitable for demanding synthesis tasks. Safety data sheets warn about corrosiveness and highlight the chemical’s affinity for releasing elemental bromine under acidic, hot, or wet conditions. Containers stress “keep tightly closed, store in a cool, dry place.” Lab workers usually understand—spilled NBS powders mean trouble for bench hygiene and, potentially, skin or lung irritation. It pays to check for fading or yellowing of sample material before use, a practice I learned quickly after once seeing a clumpy, decomposed sample spark a strong bromine odor in a fume hood.
N-Bromosuccinimide does not come from mining or plant extraction. Instead, it arises from the bromination of succinimide, a straightforward cyclic imide, using elemental bromine in a mild alkaline environment, often in aqueous or non-aqueous media. The process calls for good temperature control and careful mixing to avoid dangerous releases of bromine gas. In teaching labs, we always emphasized cooling the solution when adding bromine—exothermic reactions can spiral quickly otherwise. Once the reaction completes, it precipitates as a crystalline solid. Testing for purity often follows, using melting point checks and TLC, since impurities or incomplete bromination compromise its performance in subsequent syntheses.
NBS found its primary fame for one specific job: selectively brominating allylic and benzylic hydrogens. Chemists learned early that in the presence of light or radical initiators, NBS would step in and swap a hydrogen near a double bond or on an aromatic ring methyl group for bromine with impressive selectivity. This is how classic syntheses create intermediates for drugs, agrochemicals, or dyes. Out of personal practice, I recall the characteristic persistence required—NBS reactions don't tolerate sloppy reaction setup and demand careful monitoring of temperature, solvent dryness, and the slow addition of reagents. Later, clever modifications saw NBS involved in oxidation reactions, deprotection of groups, and even as a mild oxidant in peptide chemistry, broadening its utility well beyond the original textbook example.
N-Bromosuccinimide crops up under various names in catalogs and research articles. NBS, the common abbreviation, is almost universally understood among chemists. Other labels include NSB, succinbromimide, and bromosuccinimide. Companies often add catalog numbers for tracking, and researchers swap between these names without pause, assuming the context makes intent clear. I found early on that mislabeling in shared lab spaces can cause real headaches; double-checking both acronym and chemical structure before use avoids costly mix-ups.
Handling NBS requires good discipline in personal protection and lab protocols. It irritates eyes, skin, and lungs. Staff keep gloves, goggles, and dedicated waste containers close at hand during use. Fume hoods matter—residual bromine fumes can escape, especially if the sample contains moist air or degraded material. Fire risk remains low, but accidental mixing with reducing agents, acids, or amines triggers dangerous reactions. Manufacturers and laboratories operate with formal hazard assessments and clear signage, following both OSHA and GHS guidelines. Many institutions have anecdotal stories about poorly stored NBS producing clouds of brown vapor and triggering emergency evacuations; those lessons stick with chemists throughout their careers.
Synthetic organic chemistry is where NBS makes its biggest impact. Researchers in medicinal chemistry lean on its ability to tweak molecules with surgical precision, modifying only specific hydrogens in complex structures. Industrial chemists use it in pilot plants to prepare building-block bromides on bigger scales, particularly for polymer or pharmaceutical production. The electronics sector needs NBS for some specialty intermediates in materials science. Teaching labs introduce undergraduates to selective halogenation with NBS-based demos, a ritual almost as old as the compound itself. In my early teaching days, I saw how a successful NBS experiment could spark curiosity and confidence in new students, demystifying the complex world of controlled reactivity.
Ongoing research explores new uses for NBS and refines safer, greener ways to deploy it. Organo-catalysis, flow chemistry, and solid-phase synthesis researchers test the limits of NBS’s stability and reactivity every year. Some projects explore integrating NBS into recyclable polymer supports, hoping to cut down on waste and facilitate easy reagent recovery. New solvents and continuous-flow setups further reduce exposure risks, a trend that benefits both workplace safety and environmental impact. Journals publish dozens of new pathways every year built around NBS transformations, especially as chemists stitch together novel molecular architectures for evolving biotech and pharma needs.
Debates about NBS toxicity have a long history. Acute studies in animals point to strong irritant effects at relatively low doses, especially for respiratory and mucosal tissues. No evidence currently links NBS directly to carcinogenicity, but breakdown products—free bromine or hydrobromic acid—definitely earn suspicion. Chronic studies stay limited, due in part to the niche, rather than widespread commercial, use of the compound. Researchers support office and lab safety through substitution where possible, quick cleanup methods, and regular retraining in reagent handling. Anecdotal reports from research teams highlight skin sensitivity and occasional minor burns, emphasizing how easy it is to become complacent with something that looks so benign as a white powder.
The future looks busy for NBS. Green chemistry continues driving interest in less hazardous halogenation strategies, challenging chemists to develop more selective, less wasteful protocols. Teams work on solid-supported NBS versions, looking for ways to improve recycling and containment. Computational studies pick apart the detailed mechanisms by which NBS achieves selectivity, guiding researchers toward even smarter reagent design. I see the biggest change coming from a combination of smarter process engineering—like flow reactors—and lab culture shifts that emphasize sustainability built into every bottle of NBS delivered. The compound may be approaching its hundredth anniversary in labs, but its story continues to evolve, promising more surprises and scientific growth ahead.
N-Bromosuccinimide, known among chemists as NBS, holds a steady spot on the shelf in research and many manufacturing labs. Anyone who has ever run an organic chemistry experiment probably remembers the sharp smell and chalky look of this pale powder. Even after years away from the university lab, I still associate the name with those tense hours spent hoping for the right reaction.
Most folks encounter NBS during bromination. This reaction inserts a bromine atom into an organic molecule, and NBS specializes in targeting certain positions on carbon rings and chains. People prize this selectivity. Unlike plain bromine, which can attack several spots at once and lead to a mess of products, NBS works quietly on the side of an aromatic ring, or on the weakest carbon-hydrogen bond it finds in an aliphatic chain. Books sometimes talk about “allylic” or “benzylic” bromination, but in plain terms, this gives chemists a way to fine-tune molecules without creating a soup of unwanted byproducts.
Plenty of pharmaceuticals take shape through steps involving NBS. Some blood pressure and epilepsy drugs rely on molecules built up with precision using bromination at the right stage. It’s not just about brute-force chemistry. Controlling exactly where a bromine atom lands, and only there, helps save time and money during drug development. A more predictable outcome means fewer purification headaches down the road. I once worked on a small startup project where switching from elemental bromine to NBS cut our purification steps nearly in half, saving us days in a fast-paced launch window.
Beyond pharma, NBS backs a lot of undergraduate and graduate training. Stereochemistry problems—those three-dimensional puzzles—often get tackled with bromination as a key step. Graduate students end up learning why slow, controlled addition of NBS clears up messy reaction profiles. Over years, this hands-on training shapes better chemists, who can trust their skills in both bench work and troubleshooting.”
Despite the benefits, safety and environmental impact shouldn’t get glossed over. Powdered NBS irritates eyes and lungs, and the brominated byproducts can harm aquatic organisms if tossed down the drain. Labs handle it with gloves and goggles, but accidents do happen. Industry researchers are pushing for greener alternatives that do the same job without leaving persistent halogenated waste. Some innovative teams are redesigning these processes to use less hazardous reagents, using NBS only where nothing else works as well.
NBS still serves as an essential reagent for targeted synthesis, thanks to its blend of selectivity, efficiency, and reliability. More attention to waste management and sustainable chemistry will shape how it gets used in coming years. Chemists—both in academia and the private sector—keep searching for ways to harness the benefits of NBS while protecting both workers and the planet. That drive for smarter chemistry unfolds in every reaction set up in a flask, informed by decades of discovery and practical lessons learned in the lab.
Stepping into a chemistry lab, you might catch sight of a container labeled N-Bromosuccinimide, shortened most times to NBS. For many outside the science field, these labels seem cryptic. In reality, NBS gets a lot of action in organic synthesis, especially in research settings. Every organic chemistry student remembers the first time a textbook experiment called for this compound.
The chemical formula sums things up: C4H4BrNO2. That means four carbons, four hydrogens, one bromine, one nitrogen, and two oxygens. Lay it out, and you see a relatively simple structure. The beauty of NBS isn’t in complexity but in the way those atoms line up. The molecule packs a bromine atom onto the nitrogen of a succinimide ring, which tweaks its behavior compared to plain succinimide.
If a reaction calls for brominating a molecule—say, adding bromine to an allylic or benzylic position—NBS almost always gets the job done cleanly. Classic textbooks give countless examples: turning toluene into benzyl bromide for further transformations or modifying steroids for pharmaceutical work. The efficiency comes from NBS releasing bromine slowly in the reaction, which keeps side reactions at bay.
Not every user is a professional chemist, though. Some hobbyists and small businesses find the precision of NBS valuable. Safety always matters, because brominated compounds don’t forgive carelessness. A quick glance at the material safety data sheet spells out the risks—corrosive, possible irritant, and it needs solid ventilation any time it’s in use.
First time I handled NBS, my college professor cared less about fast results and more about not skipping the gloves and goggles. We learned that the compound can cause burns if mishandled, even in small amounts. Years later, as a lab supervisor, I still echo that message. No flashy equipment replaces basic respect for brominated reagents.
Some industries have adopted best practices that focus on airtight containers and well-maintained fume hoods. Training doesn’t stop after the first experiment—most credible labs run regular safety refreshers. Knowing the chemical formula helps, but never guarantees safe use if people overlook the day-to-day risks.
Universities and companies can boost safety by launching more accessible training resources and making sure up-to-date safety data sheets reach everyone using NBS. Digital systems to log chemical usage and incidents can catch patterns before they cause real harm. Peer review of handling habits in the lab sometimes uncovers shortcuts that, left unchecked, stack up to accidents.
N-Bromosuccinimide’s chemical formula, C4H4BrNO2, grounds it in a straightforward science. The lessons built around it stand as reminders: chemistry shapes progress, but people make or break safety. Every successful synthesis and every safe return home owes a little to knowing, respecting, and handling each chemical—NBS included—with care and purpose.
N-Bromosuccinimide, usually called NBS in labs, isn’t a household name. In chemistry, though, this reagent works hard. Anyone who’s uncapped a fresh bottle knows NBS gives off a sharp, chlorine-like odor. That strong smell comes with potent chemistry. As a brominating agent, it reacts fast and isn’t shy about it. If you walk away from an open container, you’ve got a recipe for disaster, not just ruined experiments but dangerous exposure.
Something that surprises a lot of newcomers is how quickly NBS can spoil. Heat, moisture, and light—all three encourage the bottle to break down. The white powder starts browning, clumping up, and losing its punch over several weeks if handled carelessly. That’s money down the drain for labs—plus a higher risk to anyone reaching for what looks like normal material. NBS’s breakdown products aren’t friendly, either. Exposure produces harmful fumes, potentially triggering coughing, skin irritation, and headaches. There’s no upside to sloppiness.
A chemist’s personal safety comes from good habits, not luck. The storage basics here aren’t glamorous, but they’re proven. NBS belongs in a tightly sealed container, away from sunlight and moisture. Amber glass bottles work best—plastic might leach chemicals or fail to keep out air. Drying agents, such as small bags of silica gel, help keep the environment inside the bottle bone-dry. Don’t put NBS near heat sources or in warm rooms.
The label might say “store cool,” but in real life, aim for a chemical fridge that never freezes or gets damp. Never crowd this powder beside acids, bases, or flammable solvents. Just the fumes can trigger trouble. Separation saves lives, and in this case, sanity during an emergency. If you’ve seen the cloud that rises from an NBS spill with nitric acid, you won’t store them together twice.
Decades of industry data and OSHA regulations agree that chronic exposure ramps up cancer risks. Regulations in the United States require proper hazard labeling and storage conditions for NBS. Labs must record usage and spill events, not just to stay compliant but to cut down on everyday mishaps. The American Chemical Society’s published guidelines place special emphasis on “secondary containment”—another box, bin, or tray to catch any leak or shattering bottle. If you remember a cracked glass jar leaking in a shared storage unit, you know these warnings aren’t just words on a page.
Change starts with accessible training. Nobody expects new lab staff to memorize every hazard sheet. Experienced chemists, though, can walk coworkers through examples—what a failed seal looks like, how to refresh silica gel packets, how to tell the difference between fresh and decomposed NBS. Good storage starts at the supply handoff and continues through each use, from handling with dry scoops to closing the cap every time, no exceptions.
Routine checks matter. Quarterly audits of reagent shelves, clear labeling, dated containers—all simple steps, but each one closes loopholes where mistakes love to hide. There’s no romance in safe storage, just the knowledge you’ll avoid trips to the emergency shower and keep reactions running predictably. Every careful habit with NBS pays off in reliability and peace of mind.
N-Bromosuccinimide, known to many chemists as NBS, usually sits tucked away in a brown glass bottle on the back shelf. Folks working with it know it pops up in a lot of organic syntheses. Before reaching for the bottle, knowledge beats carelessness every time. NBS carries hazards that deserve respect. Its strong oxidizing property means touching bare skin or breathing its dust isn’t just an unpleasant experience. It can burn and irritate skin, eyes, and the respiratory tract. Thinking nobody gets hurt using a small amount is a trap; even limited exposure leaves damage.
The best stories from the lab start with keeping hazardous fumes outside noses and lungs. A fume hood changes the game. Even someone with plenty of experience can underestimate vapor release or dust during weighing or transfers. Every time NBS comes out of the bottle, the sash goes down. At home or in makeshift setups, don’t even crack the seal—take it into a professional lab environment.
Nitrile gloves, safety glasses that actually cover the sides, and a sturdy lab coat offer the closest thing to armor in a chemistry lab. Cotton gloves catch on glassware and don’t block chemicals sneaking through. I’ve watched classmates peel off melting gloves or rub at watery eyes in seconds after getting too close. That stings—I’ve learned it doesn’t pay to be careless, even just grabbing a fresh bottle for inventory.
It happens to the best of us. A moment’s inattention, an elbow nudge, or a fumbled scoop dumps NBS onto the bench. Don't panic, but don’t hesitate either. Keeping spill kits for oxidizers in easy reach saves the day. Never use sawdust, paper towels, or organic absorbents, since that can cause fires with oxidizers. Scoop up the powder with a plastic tool and wash the area down with copious water—no shortcuts here. Double-bag contaminated waste and label it clearly. Chemical hygiene isn’t just about neatness; it keeps dangerous substances out of the regular trash stream and prevents unexpected reactions.
NBS doesn’t belong next to solvents, acids, or reducers. I always tuck the bottle in a dry spot, kept away from sunlight and any spot where things get warm. Sealing the lid tight keeps moisture and contaminant vapors out. Don’t transfer it to unlabeled containers, even if someone thinks it makes things look tidy. If kids or untrained folks use the same space, that bottle needs extra security.
Coffee and snacks don’t mix with NBS. Washing hands after handling it, before touching phones, doorknobs, or lunch, feels obvious but can slip anyone’s mind after a long day. Leaving gloves sitting out or stuffing contaminated tools in a drawer guarantees trouble down the line. It took a single careless moment in my old teaching lab to spread chemical dust onto three keyboards and some notes—a mess that could have been nasty if someone rubbed their eyes.
Whether new to the bench or a seasoned pro, regulations and safety data sheets shape how a lab handles NBS. No one should use it without training. A little respect works better than fear: understanding risks, following habits that keep you and your colleagues out of trouble, and remembering that shortcuts rarely save time in the long run. NBS gets the job done in a lot of cool chemistry, but only when handled with care and patience.
N-Bromosuccinimide, or NBS for short, often plays a role in transforming molecules that’s both practical and efficient. Plenty of chemists reach for it to add bromine atoms to organic compounds. Instead of dumping buckets of liquid bromine on their molecules, which can be harsh and unpredictable, NBS delivers bromine with a steady hand. The selective bromination that NBS offers makes all the difference, especially when you care about where the bromine lands.
Allylic and benzylic bromination stand out as two of the most common uses for NBS. The allylic position means the atom that sits next to a double bond, and the benzylic one sits near an aromatic ring like benzene. These positions are special because if you add a bromine there, you open the door for more chemistry to follow. Pharmaceutical chemists rely on NBS to work with pharmaceuticals or intermediates where precision matters—even one misplaced atom can change how a medicine works in the body. Even large-scale manufacturers appreciate these attributes, as safer handling and reduced waste help keep facilities efficient and workers safe.
Speed and simplicity count for a lot in a lab. NBS slices through certain transformations in a way that spares chemists from cleaning up lots of messy byproducts. That means less time purifying and more time making meaningful compounds. Brominating an alcohol to turn it into a bromide lets chemists easily switch out one group for another later. Household cleaners, dyes, and plastics use molecules born from these types of switches. NBS has a real knack for homolytic reactions, where you break chemical bonds right down the middle. This kind of transformation helps with radical bromination, letting you convert low-value starting chemicals into something far more valuable.
Even though NBS started showing up in organic labs back in the mid-20th century, it hasn't lost relevance. The reason is simple: selective means reliable. Academic papers and practical manuals list NBS frequently in stepwise syntheses. For anyone making a library of compounds—maybe hunting for the next drug or special material—using NBS can clear a direct path to your target molecule. Its solid, crystalline form travels and stores better than volatile chemicals like pure bromine, and this physical convenience plays a big part in day-to-day operations.
Working with toxic chemicals has consequences, both for people and for the planet. Safer alternatives get attention, and here NBS earns points. Compared to liquid bromine, it’s much easier to weigh and handle, so chemists dodge some serious hazards. Less chance of accidental spills, less exposure, and a lower chance of dangerous reactions going sideways all mean a safer workplace. Regulatory bodies such as OSHA and the EPA encourage these kinds of improvements, since fewer accidents and waste align with strict safety standards.
Researchers keep pushing NBS into new territories. Sometimes it helps introduce functional groups beyond bromine, working alongside catalysts or under special conditions. Scientists in green chemistry hunt for ways to recycle or replace harsh reagents, with NBS often serving as a stepping stone toward more sustainable chemistry. Ultimately, keeping sharp tools on the chemist’s bench, ones that can work efficiently and safely, lets the science move forward while lowering cost and risk. NBS is one of those tools—trusted, a bit unassuming, and quietly essential.
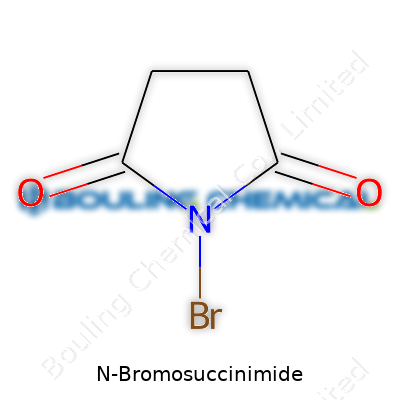
| Names | |
| Preferred IUPAC name | 1-Bromopyrrolidine-2,5-dione |
| Other names |
NBS Succinbromimide Bromosuccinimide 1-Bromosuccinimide NSB |
| Pronunciation | /ɛn-broʊ.moʊ.sʌk.ɪˈnaɪm.aɪd/ |
| Identifiers | |
| CAS Number | 128-08-5 |
| Beilstein Reference | 635873 |
| ChEBI | CHEBI:28279 |
| ChEMBL | CHEMBL1545 |
| ChemSpider | 5299 |
| DrugBank | DB11272 |
| ECHA InfoCard | 100.005.798 |
| EC Number | 1.5.5.1 |
| Gmelin Reference | 7074 |
| KEGG | C01773 |
| MeSH | D001940 |
| PubChem CID | 8255 |
| RTECS number | WN5075000 |
| UNII | VTR2H1C04P |
| UN number | UN2928 |
| CompTox Dashboard (EPA) | DTXSID3020407 |
| Properties | |
| Chemical formula | C4H4BrNO2 |
| Molar mass | 178.98 g/mol |
| Appearance | White to off-white crystalline powder |
| Odor | chlorine-like |
| Density | 1.5 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 0.9 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 9.6 |
| Basicity (pKb) | 7.95 |
| Magnetic susceptibility (χ) | Magnetic susceptibility (χ) of N-Bromosuccinimide: -76×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.565 |
| Dipole moment | 2.96 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 96.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -119.8 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin irritation, causes serious eye irritation, may cause respiratory irritation. |
| GHS labelling | GHS02, GHS07, GHS09 |
| Pictograms | GHS05,GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | Hazard statements: "H302, H315, H319, H335 |
| Precautionary statements | P210, P261, P280, P301+P312, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2-3-1-OX |
| Autoignition temperature | 355 °C |
| Lethal dose or concentration | LD₅₀ (oral, rat): 891 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 891 mg/kg |
| NIOSH | WA9485000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.1 ppm |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
Succinimide N-Chlorosuccinimide N-Iodosuccinimide |