Organic synthesis draws from a toolbox that gets bigger every decade, and N-Boc-pyrrolidine joined that box in the late 20th century as chemists looked for protective groups that actually work without too much fuss. Protecting amines in the course of multi-step synthesis has always been tricky—people once leaned on acetyl or benzyl groups, but those methods brought their own headaches with stability or removal. Boc protection, short for tert-butyloxycarbonyl, smoothed out a lot of those bumps. As protecting groups grabbed a foothold in pharmaceutical labs, N-Boc-pyrrolidine showed up more and more. Researchers didn’t cling to an academic fascination with the pyrrolidine ring; it earned a place through practicality. By the early 1990s, N-Boc-pyrrolidine found itself not just in academic journal procedures but in industrial-scale set-ups as well.
N-Boc-pyrrolidine gives synthetic chemists a reliable way to mask the nitrogen of pyrrolidine, which means opening up more reaction possibilities without unwanted side reactions. The Boc group sticks on tightly under a lot of conditions, but peels off when it’s time with a splash of acid. You find this compound as an off-white crystalline solid, easy to weigh and handle—no mess, no sticky oils. That counts for a lot on a busy bench. It stores well and does not throw surprises with the shelf life, which makes it an easy pick for research and manufacturing.
The solid form of N-Boc-pyrrolidine comes close to white, sometimes showing a faint cream tinge if you leave it out too long. Its melting point lands somewhere between 37 and 40 °C, so it sometimes softens in a warm room, but never as a liquid puddle. Once packed into an amber bottle, it holds up well, neither fumes nor gives off much odor. Its molecular formula, C9H17NO2, points to a compact structure made up of a five-membered ring and a Boc carbamate. Solubility in common organic solvents like dichloromethane and ethyl acetate helps with product work up, and the Boc group itself gives a touch of hydrophobicity, which can matter during silica gel chroma. NMR and IR spectra both show clear signs for easy identification, which means fewer doubts during purification.
Suppliers list N-Boc-pyrrolidine with purities above 98%, often tested by HPLC. Typical lots come labeled with batch numbers, CAS number 57260-72-7, and storage recommendations that just ask for a dry, cool place. On bottles used in my own work, hazard pictograms advise eye protection, and the safety data sheet calls for gloves, but this material ranks as low-hazard for most laboratory settings. TCLP (toxicity characteristic leaching procedure) does not put it in a flagged waste category, so disposal in regular organic waste suits most labs. Product comes in amber vials and, at scale, in HDPE drums for those running big reactions.
Making N-Boc-pyrrolidine usually involves reacting pyrrolidine with di-tert-butyl dicarbonate (Boc2O) in the presence of a base such as triethylamine. The base keeps things moving along by mopping up acids made during the reaction. Sometimes DMAP pops in as a catalyst. Work-up steps remain simple—extract, wash, and dry—since the byproducts stay easy to separate. The biggest hassle comes from making sure you keep water out. Any moisture prompts unwanted hydrolysis. The prep suits both milligram and kilogram jobs, so pharmaceutical scale-up doesn’t require a shift in reaction logic.
The Boc-protected pyrrolidine acts as a stable amine surrogate, letting the nitrogen sit tight through grueling steps like alkylation, acylation, and cross-couplings. You can tack on side chains at the 2, 3, or 4 positions using lithiation chemistry, and the Boc protection holds together under basic and most neutral conditions. Cleaving the Boc group gets as easy as treating with trifluoroacetic acid or HCl in dioxane—about a two-hour job in my own hands. After removal, the parent pyrrolidine pops out, ready for further transformation without leftover carbamate mess. Adding substituents after Boc removal gives good yields, which matters for building complicated target molecules like many drug scaffolds.
Anyone scanning catalogs searching for Boc-protected pyrrolidine stumbles over several names: tert-butyl 1-pyrrolidinecarboxylate, 1-Boc-pyrrolidine, and just N-Boc-pyrrolidine. The CAS number 57260-72-7 helps dodge confusion. Sometimes product comes flagged as “carbamic acid, 1-pyrrolidinyl-, 1,1-dimethylethyl ester” in regulatory filings, which looks clunky on a label but matches its structure precisely.
Handling N-Boc-pyrrolidine doesn’t give many problems if you stick to gloves and goggles. I don’t recall any of my colleagues reporting skin sensitivity or respiratory issues. It doesn’t splash or fume under normal bench conditions, so standard good lab practice covers it. For big reactors, operators should always check for basic ventilation. Cleaning up spills needs a dry, lab-grade absorbent—never water—because you want to prevent hydrolysis or slippery messes. Waste solutions handle easily with non-halogenated organic solvents but keeping a log of disposal keeps the safety office happy.
Drug synthesis takes up the biggest share of N-Boc-pyrrolidine’s use. Medicinal chemists design and tweak pyrrolidine-containing drugs for targets like antiviral and central nervous system applications. The Boc group lets teams run combinatorial chemistry without worrying about side reactions. Material chemists also fit the molecule into polymer research, testing ring-containing units for elastic properties or adhesion. Its presence shows up in peptide chemistry, organic electronics, and—more recently—fragment-based drug discovery, where small, robust motifs go a long way. Custom chemical companies keep it in bulk for just this kind of versatility.
In the context of early-stage R&D, N-Boc-pyrrolidine gives synthetic routes the breathing room they need. Its resistance to a lot of reaction conditions means teams can plan longer synthetic cascades without panic over amine reactivity. In my own project flow, having a Boc-protected nitrogen has rescued more than one late-stage route from failure. As automated synthesis robots grow, Boc groups have become practically standard—machines operate more easily with fewer side-reactions. Publications in journals like JOC or Organic Letters keep reporting new modifications starting from this intermediate, proving its staying power.
Toxicological profiling for N-Boc-pyrrolidine itself does not rank as a serious concern, but regulatory teams still check for Boc-derived byproducts in finished pharmaceuticals. The compound’s low acute toxicity and predictable metabolism profile make it safer than a lot of reagents on the organic bench. Of course, once cleaved to plain pyrrolidine, toxicity rises a bit—pyrrolidine itself acts as a mild irritant, and keeping hands away from your face remains rule number one. Regulatory agencies watch for traces of Boc-cleavage products in final APIs. So far, industry data do not show evidence for bioaccumulation or carcinogenicity at the levels typically encountered in the lab.
N-Boc-pyrrolidine’s use will likely keep growing as drug hunters chase new amine motifs in neuroactive agents, and as automated chemistry gets even bigger. Efforts to engineer greener Boc-cleavage protocols—less acid, more recycling—stand on the horizon. In my experience talking to scale-up teams, better methods for recycling Boc2O and tightening yields matter just as much as discovering a brand new reaction transformation. Late-stage functionalization techniques should open doors for more selective modification of the pyrrolidine ring, but every new trick that works with the Boc-protected version helps big pharma shorten synthetic plans. With its role so cemented in med-chem and method development, demand looks set to grow as long as chemists keep chasing new molecular shapes.
Talking about N-Boc-Pyrrolidine, a purity number like 98% or 99% does more than fill out a technical detail. In chemical labs, purity shapes results. If the purity skips even a half percent, you can see effects—some barely matter, some trash a full month’s effort.
I remember my first job handling small-scale syntheses. The manager handed me a bottle labeled "N-Boc-Pyrrolidine, 98% min." He said, "You won’t notice it until you run into something unexpected." And sure enough, a bit of extra water or a forgotten side-product popped up as streaks on TLC, and our next step turned from predictable to stubborn. No chemistry undergrad understands the headache of chasing down a mystery impurity until they've had a column drag on forever—all because the starting material didn’t match the spec.
Most suppliers stick with 98% or above for research chemicals like N-Boc-Pyrrolidine. Some might throw in a 99% version, but prices shoot up. That jump isn’t just about profit. Squeezing out that last percent can take days of careful distillation or fancy purification. Sometimes the extra step is charcoal filtering, sometimes it’s automated chromatography with a robot more expensive than most cars.
I’ve seen people ignore a dirty batch because it sort of looked fine. Then, nothing reacted quite right in the next round. Small impurities can tie up your catalyst, ruin yields, or sneak through as nasties in drug candidates. In early discovery work, you can sometimes get away with a bit of grime, but anyone scaling up or making something heading for animal tests starts sweating those decimal places.
Labels and certificates look clean, but nothing replaces checking with your own eyes. Thin-layer chromatography, HPLC, or NMR—these tools keep chemists honest. Some batches labeled "pure" actually pack in 1–2% of similar gunk or leftover solvent. One supplier might sell "98%" material spotless except for some water; another might hide a hard-to-remove isomer. I learned to ask for chromatograms, not just a number. Sometimes, it means arguing with purchasing, but cutting a corner once burned me on a hundred grams of material that couldn’t hit the benchmark.
Pharmaceutical development has it even tougher. Here, regulatory rules demand figures like 99.5% or better, along with lists showing what the minor contaminants are. No escape from testing at every step. In small academic labs, it’s tempting to trust the vendor. In contract research or manufacturing, nobody trusts—each lot gets a test. Impurities aren’t just a nuisance—they spark lawsuits, recalls, or, in the worst cases, health problems for patients.
Pick a reputable supplier. Don’t take a webstore’s word for it—ask for batch analysis or test the material yourself. Spend more if your result depends on it. Try running a TLC or quick NMR to check you’re starting with the right stuff. When using N-Boc-Pyrrolidine for an important project, purity offers peace of mind that your experiment really tells you what you want to know. If money’s tight, buy smaller amounts rather than a discount drum with a question mark hanging over it. Every chemist I know learns this lesson—some the easy way, most the hard way.
N-Boc-Pyrrolidine isn’t as high-profile as some chemicals you might hear about, but ask any lab tech or chemist how much it matters to keep it in good shape. You don’t want pricey intermediates getting ruined. This compound turns up a lot in organic syntheses, and an unstable batch can mess with a lot more than just one experiment.
Most folks who regularly handle N-Boc-Pyrrolidine will tell you: keep it cool and dry. Leaving this compound out on the bench invites trouble. Too much heat can send it breaking down faster than you’d expect. Moisture opens the door to hydrolysis – which you definitely don’t want. A lot of people keep their bottle capped tight and stash it in a dedicated chemical fridge, usually set below room temperature.
Light exposure sometimes gets ignored. Extended light can mess with a chemical like this, especially on a hot summer day if it’s near a bright window. Opaque bottles or dark glass cut down those risks. I learned the hard way once, walking in after a weekend to find a container tinted yellow, with the contents sticky and half gone. Nothing cuts productivity like ruined stock.
Clear labeling isn’t just a formality. N-Boc-Pyrrolidine looks harmless enough, but without proper storage, the container can build up unpredictable pressure, and it doesn't always smell it until things have gone wrong. Good practice is to include not just the date but details on storage temp and humidity targets. If the lab has a log, jot every movement down. This way, if something goes off, you don’t spend your afternoon hunting through questionable vials.
I’ve seen new students grab the big communal desiccator off the shelf, thinking it’s good enough for all dry storage. Problem is, unless you replace drying agents often—and check for leaks—even a desiccator loses its bite. Dedicated cabinets help, but only if staff commit to keeping clutter down and checking seals regularly.
Sometimes you have more compound than you’ll use in a month. In those cases, you can split up the bulk into smaller sealed aliquots. That limits how many freeze-thaw cycles the whole batch sees. Putting a silica gel packet inside the storage container never hurts. For deep storage, I’ve had good luck with airtight vessels in low-humidity freezers. Most reputable vendors ship N-Boc-Pyrrolidine with enough stabilizer for shipping, but if in doubt, ask for the data sheet. Sometimes, keeping things right means knowing what you’re up against before you even pop the cap.
Messing up storage can turn a $200 bottle into hazardous waste in no time. With university budgets always stretched thin, you only make that mistake once. Paying attention to light, air, and moisture makes a difference. Higher purity translates to better yields and fewer headaches down the road. If storage goes wrong, you can’t always fix it with fancy chemistry later. Prevention works better than any remedy I’ve tried.
N-Boc-Pyrrolidine may not seem like much, just another flask on the shelf, but keeping it safe boosts productivity as much as any fancy equipment in the lab. Good habits now mean you don’t spend tomorrow cleaning up last month’s sloppy shortcuts.
Step into many chemistry labs, and you'll spot small bottles marked with names that look cryptic at first glance. N-Boc-Pyrrolidine is one of those that pops up often. It’s not just some exotic reagent sitting on the shelf; plenty of folks in both academia and industry reach for it regularly. N-Boc-Pyrrolidine comes into play, especially during the creation of molecules for pharmaceuticals and some agricultural products. Blocking groups like the Boc (tert-butyloxycarbonyl) help chemists control which parts of a molecule react, almost like using painter’s tape to keep a trim line sharp on a wall. That sort of control doesn’t just save time — it cuts down on do-overs and wasted resources.
The pharmaceutical industry burns through thousands of reactions daily, searching for the next promising drug. N-Boc-Pyrrolidine’s main calling card shows up here. Medicinal chemists protect the nitrogen on pyrrolidine with a Boc group so it doesn’t jump into the action too early. Later, after building up a more complex molecule, they remove the Boc to reveal the reactive amine exactly when needed. This sequence allows careful construction of everything from antivirals to treatments for neurological disorders. Options open up: researchers can introduce new features, attach complex side chains, and try out novel arrangements that could become the backbone of a future medicine.
Anyone who’s worked with peptides knows how fussy amino acids can get. Peptide synthesis demands patience and reliable protecting groups. N-Boc-Pyrrolidine acts like a puzzle piece that slots in cleanly with other amino-protected reagents. In practice, using the Boc group on pyrrolidine means that the chain assembly moves forward with fewer unexpected hiccups. The final products find homes in research, drug design, or therapeutic protein work. Because so much depends on keeping amines safe until the right step, N-Boc-Pyrrolidine keeps things rolling smoothly in a process where mistakes get expensive fast.
Outside of pharma, N-Boc-Pyrrolidine still holds a place among specialists tackling building blocks for dyes, fragrances, and crop-protection chemicals. Those multi-step recipes for advanced materials often depend on clever protection strategies. Using this compound, chemists make sure functional groups only react when it benefits the overall design. Take an example in asymmetric synthesis: having control over nitrogen reactivity helps create compounds in just one hand (chiral form), which can matter a lot in flavor chemistry and pesticides. Market pressures push for cleaner reactions with less waste, and N-Boc-Pyrrolidine has helped some processes cut down on hazardous byproducts and unnecessary purification steps.
Easy access and scale-up of N-Boc-Pyrrolidine let small labs and large companies benefit alike. Still, cost and waste handling pop up as worries, especially with protecting groups that add extra steps. Some groups tinker with “greener” chemistry, searching for ways to swap out solvents or streamline the removal of Boc groups without adding harsh conditions. Continued improvements in supply chain reliability and process optimization could help make chemistries relying on N-Boc-Pyrrolidine run smoother and cheaper. The steady demand shows no sign of slowing, so folks invested in process chemistry keep their eyes on new routes that promise less mess every step of the way.
Spend any time in a chemistry lab, and you run into the same puzzle over and over: picking out the right bottle, working with substances that only make sense on paper. Sometimes, it’s not the label but the little things tucked away in a database that matter most. N-Boc-Pyrrolidine, which carries the chemical name 1-Boc-pyrrolidine, comes with its own tag: CAS number 57260-71-6. Sounds simple, right? Digging a bit deeper, this string of numbers stands out as something more than just bureaucracy or a paperwork requirement.
A chemical’s CAS number works like a fingerprint. My own chemistry grad-school years involved walking the tightrope between similar-sounding compounds. Pick up the wrong one? That’s a wasted week or a failed experiment, if not worse. That’s where the CAS number steps in—a direct line to exactly what’s inside the bottle. For N-Boc-Pyrrolidine, 57260-71-6 isn’t just for paperwork. It’s the reason a researcher knows they’re about to run a reaction with the right protected pyrrolidine instead of an unrelated amine.
Outside the lab, think about the folks managing regulatory paperwork. Import a chemical across borders, and authorities don’t care about company nicknames or trade names—they want that CAS number. Even suppliers use it as common ground so scientists in Tokyo and New York talk about the same thing.
Countless stories float around about mix-ups caused by ambiguous names. In my experience working with contract research organizations, project delays often trace back to basic ID errors. N-Boc-Pyrrolidine gets used in pharma as a building block, for example in synthesizing drug candidates. One digit off on a label, and the whole batch can go sideways—thousands of dollars and months of work, all because a clear system wasn’t followed.
Chemical safety data sheets, procurement forms, and research articles all hinge on clarity. Without a standard ID, things drift into messy territory. Imagine a lab tech, late at night, hunting supplier catalogs. CAS 57260-71-6 saves time, reduces risk, and helps everyone stay grounded in reality instead of jargon.
With tons of companies selling the same substance under different names, standardization carries real weight. CAS Registry Numbers, run by the American Chemical Society, step in where language fails. For something like N-Boc-Pyrrolidine, the CAS number smooths out confusion between “tert-butoxycarbonyl pyrrolidine” and similar-sounding cousins.
Even the rise of online chemical marketplaces hinges on this numbering system. During the last few years managing chemical inventories and ordering, I came to rely on CAS numbers more than catalog IDs or local codes. This system, which covers millions of chemicals now, works because scientists, customs officers, and purchasing agents all agree on one reference.
To keep scientific progress steady, everyone from students to suppliers should start with CAS numbers. Training new lab folks on checking these identifiers makes things smoother in the long run. On the software side, chemistry databases could flag near-matches on CAS numbers to catch ordering mistakes before they happen.
The problem isn’t the chemical complexity—it's human error and loose ends. Anchoring chemical identity in simple, agreed-upon numbers like 57260-71-6 means better research, less waste, and safer outcomes for everyone who touches a bottle, from factory floor to fume hood.
Ask any chemist who has tried to track down specialty intermediates about their most memorable frustrations, and sourcing N-Boc-Pyrrolidine in decent amounts usually makes the list. This compound turns up everywhere in pharmaceutical manufacturing and organic synthesis, acting as a sturdy building block. But dreams of getting it fast and cheap fizzle out the minute you try to push past lab-scale bottles and ask for real volume.
Pharma and biotech companies gobble up N-Boc-Pyrrolidine. Every startup looking to tweak a molecule for better bioavailability or IP protection eyes it as a favorite route to new heterocycles. It props up peptide chains and lets chemists sidestep messy protecting group work. It gets requested, then suddenly hundreds of grams, or kilos, vanish from shelves as word spreads about a new project direction.
With rising patent cliffs, generics producers are rolling out their own versions of blockbuster drugs. Many of them quietly depend on intermediates like this one. Even students with grant money at university labs feel the pressure. They call suppliers, send quotes, and sometimes get ghosted when asking for more than a few bottles at a time.
Factories aren’t short of reactors, but most don’t want risk unless they see solid demand. Making a thousand kilos of N-Boc-Pyrrolidine takes expensive starting materials, clean equipment and skilled hands who understand what can go wrong—especially since this reaction isn’t completely forgiving. Regulatory roadblocks in some regions only add to the headaches. The result? Most distributors keep smaller stock, shifting the burden to custom synthesis firms, who ask for longer lead times and higher prices.
COVID didn’t help. Shipping lanes turned choppy, and supplies of everything, from glassware to raw chemicals, got tangled up. What used to be five weeks in normal times stretched to four months, and prices bounced around with every new disruption in the supply chain. Big pharmaceutical companies with annual contracts can handle it, but small companies and academic labs sometimes run out of what they need in the middle of a project.
In my experience, long-term reliability beats a quick bargain. Building a relationship with a few trusted suppliers—or working closely with custom synthesis companies—cuts through the red tape when things get tight. Labs get stuck if they rely on last-minute ordering, so planning and forecasting can make all the difference, even when money’s tight. Group purchasing helps as well. When universities team up for bulk buys, or small pharma companies band together in purchasing consortiums, suppliers start to take notice.
Also, some researchers now look at alternative protecting groups, or tinker with synthesis routes that avoid hard-to-find intermediates like N-Boc-Pyrrolidine. That takes more upfront work, but reduces long-term risks. For those tied to classic chemistry, working out a regular schedule with vendors, and locking in price and supply agreements in advance, clears up headaches—provided you’ve done the legwork comparing sources based in different countries.
Bringing N-Boc-Pyrrolidine in bulk to the lab or plant floor hasn’t gotten any easier with rising demand, global disruptions, and tough regulations. Navigating this market means thinking a few steps ahead, balancing science with logistics, and leaning on old-fashioned relationships just as much as reaction mechanisms.
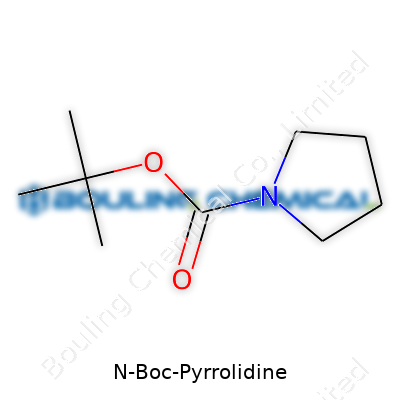
| Names | |
| Preferred IUPAC name | tert-butyl 1-pyrrolidinecarboxylate |
| Other names |
tert-Butoxycarbonyl pyrrolidine N-Boc-pyrrolidine 1-Boc-pyrrolidine N-(tert-Butoxycarbonyl)pyrrolidine |
| Pronunciation | /ɛn bɒk paɪˈrɒlɪdiːn/ |
| Identifiers | |
| CAS Number | 79955-52-9 |
| 3D model (JSmol) | N-Boc-Pyrrolidine (tert-Butoxycarbonyl-pyrrolidine) – JSmol string: ``` CC(C)(C)OC(=O)N1CCCC1 ``` |
| Beilstein Reference | 3441166 |
| ChEBI | CHEBI:131381 |
| ChEMBL | CHEMBL398042 |
| ChemSpider | 75525 |
| DrugBank | DB08375 |
| ECHA InfoCard | CINFOCARD00019345 |
| EC Number | 1336282-47-1 |
| Gmelin Reference | 153892 |
| KEGG | C13972 |
| MeSH | D000070824 |
| PubChem CID | 127329 |
| RTECS number | UE3542500 |
| UNII | 0L85Q367SU |
| UN number | 3272 |
| CompTox Dashboard (EPA) | DTXSID4041515 |
| Properties | |
| Chemical formula | C9H17NO2 |
| Molar mass | 143.21 g/mol |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | 1.03 g/cm3 |
| Solubility in water | Insoluble |
| log P | 1.51 |
| Acidity (pKa) | pKa = 11.3 |
| Basicity (pKb) | 2.68 |
| Magnetic susceptibility (χ) | -62.97·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.462 |
| Viscosity | Viscosity: 0.902 cP (20°C) |
| Dipole moment | 4.86 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 247.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -5897.2 kJ/mol |
| Pharmacology | |
| ATC code | |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | Precautionary statements: "P261, P305+P351+P338 |
| NFPA 704 (fire diamond) | Health: 1, Flammability: 1, Instability: 0, Special: - |
| Flash point | 87°C |
| PEL (Permissible) | Not established |
| REL (Recommended) | 15 to 25°C |
| Related compounds | |
| Related compounds |
Pyrrolidine N-Boc-piperidine N-Boc-azetidine N-Boc-morpholine N-Boc-pyrrole |