Organic synthesis owes plenty to the humble pyrrole ring, but protecting groups have shaped much of the story. Back in the 1970s and 80s, chemists often struggled to keep heterocycles free from side reactions, so protecting nitrogen was a big challenge. Out of this pressure came N-Boc-Pyrrole, with Boc standing for tert-butoxycarbonyl, a group borrowed from peptide chemistry. Adding Boc protection to pyrrole was no simple leap—it took tinkering, plenty of failed runs, and even more patience refining reaction conditions. Labs found out quickly that Boc groups came off neatly under mild acid, sparing them from harsh conditions and letting them carry out multi-step syntheses without breaking step. N-Boc-Pyrrole brought an extra layer of flexibility for researchers who wanted to manipulate pyrrole chemistry without getting tangled up in by-products.
N-Boc-Pyrrole looks like a pale yellow oil in most bottles. It's a building block people in pharma and agrochemical labs pull off the shelf when they want to tuck away the reactive nitrogen on a pyrrole, carry out tough reactions, and then bring the original ring back. It's far from flashy, but it shows up in workhorse steps wherever selectivity matters more than speed. Some suppliers offer it in bulk, others cap their bottle sizes. The stability under standard storage makes it less of a diva than other nitrogen-protected compounds, which in real-world terms means fewer headaches and phone calls to technical support when inventory sits for months.
This compound’s molecular weight clocks in around 167 g/mol. It carries the standard features of protected heterocycles, with a faint odor and an oily texture. At room temperature, you won't usually see any rapid decomposition unless light and moisture sneak in. The boiling point floats just above 120°C at reduced pressure, and solubility isn’t much of an issue in common organic solvents like dichloromethane or ether—something synthetic chemists actually care about in a real flask. The Boc group acts like an umbrella, shielding the nitrogen lone pair and taking the sting out of unwanted side reactions that otherwise dog the synthesis of more delicate intermediates.
Regulations and good lab practices expect N-Boc-Pyrrole to be labeled with its CAS number, 13160-11-3, and structural details so users aren’t guessing what they received. Labels often show purity, typical lots range from 95% to 99%. Water content, color, and storage conditions come printed right on the bottle, not buried in some server half a continent away. Companies that care about their long-term clients provide detailed certificates of analysis. Hazards warnings get their own space, so no one feigns ignorance about skin or respiratory risks. Precise specifications simplify compliance audits and pull policies away from grey areas.
Making N-Boc-Pyrrole usually starts with treating pyrrole with di-tert-butyl dicarbonate in the presence of a base like triethylamine. Chemists toss everything into a dry organic solvent, typically dichloromethane, and let the reaction run under a nitrogen blanket. Workup means routine aqueous extraction and evaporation, followed by purification using column chromatography. Some labs use solid-supported reagents or switch solvents to dimethylformamide for better yields, but most routes circle back to the same basic operation. The process stands as a robust method that adapts well to scale-ups and tinkering with substituents, which matters more in industrial pipelines than academic paperwork.
People exploit N-Boc-Pyrrole because it keeps the nitrogen safe through a long gauntlet of reactions—metalations, cross-couplings, even bromination at the 2-position go smoother with the Boc present. That lets chemists tack on groups for drug discovery or build new scaffolds for advanced materials. Acidic deprotection using trifluoroacetic acid or hydrochloric acid unhooks the Boc group, leaving pyrrole behind, ready for further chemistry. Reductions, oxidations, and alkylations all proceed without the nitrogen fighting back, so labs can tweak the skeleton as much as the route demands.
Alongside N-Boc-Pyrrole, you’ll see labels like 1-(tert-Butoxycarbonyl)pyrrole, Pyrrole, N-(tert-butoxycarbonyl)-, and tert-Butoxycarbonylpyrrole. Chemical catalogs still use all of them interchangeably, so checking the molecular structure in catalog entries or SDS sheets often saves a lab from ordering the wrong product—mix-ups waste money and time, both tight commodities during grant season. Cross-referencing CAS numbers short-circuits confusion. Smaller suppliers sometimes lean on brand names, but the practical core remains untouched: people want the protected pyrrole, no matter the tagline.
Working with N-Boc-Pyrrole calls for vigilance—skin contact leads to irritation, and prolonged inhalation can cause mild respiratory issues. While it’s not as hazardous as many nitrogen reagents, people still use gloves, lab coats, and chemical fume hoods. Waste disposal lines up under organic solvent protocols; most institutions require collection for incineration instead of flushing it down the sink. Regulatory frameworks like GHS place it under irritant categories, and compliance officers expect up-to-date documentation in every corner of the lab. Regular training keeps accidents rare, but real-world experience shows complacency creeps in if teams go months without reminders or safety drills.
Pharmaceutical research stands out as the main field using N-Boc-Pyrrole, especially for synthesizing bioactive molecules and prepping advanced drug-like scaffolds. Med chemists rely on Boc protection for building libraries quickly and picking routes that scale cleanly from milligrams to kilograms. Agrochemical R&D, material science, and dye synthesis also draw from the same basic chemistry, showing just how much mileage comes from a single functional group. Research into light-responsive molecules and polymer building blocks pulls N-Boc-Pyrrole into more esoteric territory, confirming its place outside big-pharma headlines.
A wave of recent work uses N-Boc-Pyrrole as a launch point for heteroarene modifications and cross-couplings that weren’t routine even a decade ago. High-throughput synthesis, automation, and robotics now interface with protected pyrrole chemistry, letting groups build vast compound libraries with retained activity and better patent positions. Projects focusing on photoswitchable compounds and targeted molecular delivery build from the protected ring, which absorbs damage and lets chemists pivot in the face of side reactions. Time after time, the field returns to N-Boc-Pyrrole because new routes and strategies still benefit from its stability and predictable reactivity.
Concerns about toxicity usually revolve around the parent pyrrole more than the Boc-protected derivative, though animal data remain sparse. Some in-vitro studies hint at moderate cytotoxicity when concentrations rise far above practical working levels. Teratogenicity and chronic exposure assessments haven’t flagged substantial dangers under standard lab practices, but regulatory agencies always encourage ongoing data collection. In my own experience, routine use over years in teaching and research labs rarely brings accidental poisonings, but the penalties for ignoring safety gear remain steep if local exposure limits see a breach. Until more is known, common sense and standard protective equipment form a solid first line of defense.
The value of N-Boc-Pyrrole climbs as synthetic demands rise. Green chemistry projects demand cleaner, less energy-intensive deprotection steps; new protecting groups compete, but Boc still holds its ground with its mild removal under acid. Automated flow systems have begun to adopt Boc-pyrrole reactions thanks to solid, predictable outcomes. Combinatorial chemistry, especially in early-stage drug discovery, calls for protecting groups that lift off easily without dragging unwanted fragments along. The coming decade could see engineered strains or biocatalysts handle Boc-protected pyrroles, fast-tracking scale-ups and trimming reliance on harsh reagents. People look for sustainable supply chains, and industry shifts incentives toward vendors that guarantee tighter specs and traceability all the way back to the starting material. On the front lines where hands meet glassware, familiarity, versatility, and reliability keep N-Boc-Pyrrole a fixture in every well-stocked synthesis lab.
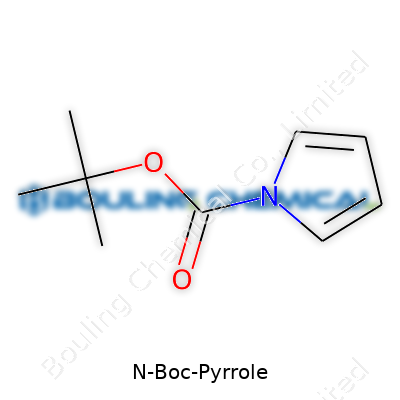
N-Boc-pyrrole stands out among laboratory chemicals. This compound features a pyrrole ring dressed up with a tert-butoxycarbonyl (Boc) group on its nitrogen atom. For those who want to see its face, the structure features a five-membered unsaturated ring with one nitrogen, thanks to that classic pyrrole base. Then, hanging off the nitrogen, you find the Boc group—a bulky, carbamate-style shield with the formula (C5H9)CO2—stuck on. Conceptually, it’s sort of like cloaking the reactive nitrogen to prevent it from jumping into unwanted reactions, which happens often in organic synthesis.
The Chemical Abstracts Service (CAS) number for N-Boc-pyrrole is 99199-60-7. This number links directly to its identity, not just for chemists but for suppliers, safety data sheets, customs forms, and all sorts of logistical hurdles one typically meets before a reaction even begins.
Pyrrole alone gets pretty feisty during chemical reactions. Free nitrogen usually grabs protons and loves to make new bonds. That’s handy for some reactions, yet a nightmare for others. That’s where the Boc group comes in, throwing a big, insulating umbrella over the nitrogen. This protection prevents side reactions and lets you fiddle with the other positions on the ring without worrying about the nitrogen betraying your plans midway. A chemist can use strong bases, explore plenty of reactions, and still count on the nitrogen to keep quiet until the moment comes to remove the Boc group and let it loose again.
Many in organic synthesis—and I’ve dealt with more than a few—treat N-Boc protection as a sort of insurance policy. The structure’s stability, especially in the face of acids, gives room to breathe. The Boc group steps out easily with a jolt of acid, making it possible to reveal the bare pyrrole at the very end. Timing matters, especially with complex targets. Having protection on demand means fewer failed syntheses and more predictable outcomes.
In pharmaceutical labs, N-Boc-pyrrole proves essential for building new compounds, especially for the regulatory dance around heterocycles. It helps put together key fragments without the need for constant patch-up work. For universities and industry, this sort of building-block molecule saves batches of time and money—not least because it keeps reactions cleaner. Less troubleshooting, less purification, and fewer headaches at the end of a long day.
I’ve watched people in research scramble with unprotected pyrroles, only to see yields tumble and impurity profiles balloon. You bring N-Boc derivatives into that same setup, and the headaches shrink. Instead of battling the molecule for control, you get to focus on the creative parts of synthesis. Some of the new drug molecules in clinical trials today owe their existence, in no small part, to reliable protection strategies like this one.
Getting consistent batches of N-Boc-pyrrole depends a lot on supply chains and the care taken in storage. This molecule holds up well if kept cool and dry, away from strong acids or bases. Sometimes suppliers cut corners, and folks end up with degraded material. It pays to double-check certifications—not just a CAS number, but genuine analysis. More access to reputable chemical vendors and better chemical tracking systems could make life easier for research labs. Shoddy chemicals cost time, money, and sometimes bring entire research projects to a halt.
Step into any organic chemistry lab, and among the crowd of glassware and colored liquids, you’ll often spot a few vials labeled “N-Boc-Pyrrole.” For chemists, this compound is more than a tongue-twister—it solves plenty of real problems that crop up during synthesis. Early in my research career, I tangled with pyrroles that just wouldn’t behave. They were too reactive, too sticky, too quick to join the wrong partner at the wrong time. That’s where N-Boc protection earns its pay.
Chemists use N-Boc-Pyrrole to “mask” the pyrrole nitrogen. Pyrrole loves to react at the nitrogen, often getting tied up in side reactions nobody wants. Slapping on a Boc group lets the rest of the molecule play ball without interference. It’s like letting the kids run in the yard while one stays in for a nap—less chaos, more control.
As reactions unfold, the Boc group keeps the nitrogen tucked away. When all the heavy lifting is done, a quick splash of acid sends the Boc flying off, revealing the original pyrrole ready for final tweaks. I’ve worked through plenty of multistep syntheses, and having a smart protecting group like this saves wasted effort and resources.
N-Boc-Pyrrole moves chemistry forward, especially in drug and natural product synthesis. Pyrrole units crop up everywhere you look—from porphyrins in your blood to new experimental cancer drugs. Building these big, complicated molecules means you need a smart toolkit. N-Boc-Pyrrole lets chemists bolt on extra fragments without tripping over unwanted reactions. I’ve seen it put to work in cross-coupling reactions, letting us string together fragments that eventually become part of a larger, biologically active structure.
Solid-phase peptide synthesis also calls on N-Boc-Pyrrole. This setup allows researchers to engineer small molecules and peptides for probing cell function, drug targets, or even to make new kinds of organic materials.
Making new heterocycles often starts with pyrrole or a close cousin. From there, using N-Boc-Pyrrole opens doors for putting substitutes or functional groups only on certain spots—a trick that proved tricky before. I once struggled with controlling substitution on an unsubstituted pyrrole ring. Switching to the Boc-protected version gave me a better handle and made purification less of a nightmare, because the product stays cleaner through the reaction sequence.
Plain pyrrole stinks, stains, and sometimes irritates the skin. N-Boc-Pyrrole gives an easier time in the lab. It's more stable and far less prone to evaporation. In scaling up reactions, small savings in safety and handling compound quickly—nobody wants a surprise runaway reaction.
N-Boc-Pyrrole’s value feels obvious once you’ve wrestled with unprotected pyrrole derivatives. Researchers look for new protecting groups, but the Boc group strikes a balance between being tough enough for rough chemistry and easy to remove at the end. Down the road, greener and even more efficient alternatives might tip the balance, but for now, this compound has carved out its place as a reliable teammate in many projects.
I’ve handled plenty of reagents in the lab that look harmless but turn into a real headache after a week on the shelf. N-Boc-pyrrole fits right in with this crowd. It's a handy protecting group for chemists aiming to build new molecules or tinker with heterocycles. Still, the stuff carries its own quirks, and if you slack on storage, you’ll end up with junk instead of a usable reagent. You don’t need twenty years in the field to notice: ignore proper storage, and purity fades quick, meaning failed reactions and wasted time.
Plenty of chemists—myself included—get too comfortable with common reagents and ignore the fine print on datasheets. Few remember the wasted mornings figuring out why a reaction played dead, only to discover N-Boc-pyrrole had kicked off a week earlier due to sloppy storage.
Light and air get a lot of blame for ruining chemicals, but moisture and high temps do the real damage with N-Boc-pyrrole. I store mine in a tightly sealed glass bottle, parked in a fridge around 2-8°C. Room temperature works for very short stints, but anything longer and you risk hydrolysis breaking apart the Boc group, and then you're left with a plain old pyrrole. Even a few hot days at 25°C can reduce shelf life, especially if humidity creeps into the container. The difference in outcome between following and ignoring these details isn't small—impurities add up, and reactions start to sputter or twist in weird directions.
Nobody likes the idea of crawling back and repeating syntheses, especially when troubleshooting points to degraded N-Boc-pyrrole. It's a lesson I learned early on: the cost of an extra dehumidifier or a few desiccant packs in the storage cabinet pays off after the third or fourth time you see a clean TLC plate and the yield matches your notebook.
Most folks tackle stability challenges by doubling down on dryness. Silica gel or molecular sieves work well, especially in those glass-top jars you can find cheap online. Don’t overlook these tricks. Even small spikes in local humidity—door open for too long, or reagent left out during breaks—lead to micro-level hydrolysis over time. Using small aliquots helps, too: take what you need, keep the rest sealed. I label each portion so nothing lingers outside the main container, and combine this with regular checks on the reagent state using NMR or HPLC when I spot discoloration or an off odor. You won't find big chemical companies tossing their N-Boc-pyrrole around loose, and there's a reason for that.
You wouldn't want broken-down N-Boc-pyrrole near finished pharmaceuticals, not just for yield but for trace impurities sneaking through to the final product. Though N-Boc-pyrrole doesn't top the charts as a health risk, keeping degraded material out of the mix makes a big difference, whether you work in drug development or academic labs. Avoiding careless disposal—or worse, stockpiling expired reagent in the back of a cabinet—reduces both waste costs and the risk of contamination.
Having a tidy system for tracking received batches and open dates saves a lot of second-guessing. Regular reviews of inventory keep old material out, new material properly stored, and lab work humming along. Spending a few extra minutes on these steps makes a world of difference to the reliability of your chemistry.
I’ve spent long hours in labs, and every time a bottle with a complex name like N-Boc-Pyrrole lands on the bench, there’s a familiar checklist that goes through my mind. N-Boc-Pyrrole finds its place in organic synthesis, often as a protecting group for functional groups in larger molecules. The thing is, even simple-sounding reagents can pack hidden risks, and pretending otherwise ends badly for everyone nearby.
Gloves and safety glasses aren’t just a formality. This compound can irritate skin and eyes on contact, and you’ll know quickly if you spill or splash it. Short sleeves or bare hands invite trouble and could mean nasty rashes or stinging eyes. Good nitrile gloves stand up well—don’t bother with thin latex. Those splash goggles you left in your drawer? Now’s the time to dig them out.
Even the sharpest chemists get casual about air quality after a hundred syntheses. Still, N-Boc-Pyrrole can release vapors, especially under heat or if you spill it. I’ve seen colleagues regret leaning in close to pipette or sniff a sample. Always work under a fume hood. That fan overhead does more than drown out small talk—it pulls away vapors before you inhale them. Good ventilation is insurance you pay once that keeps giving.
Splatters and spills never happen on purpose. Speed matters more than panic. Absorbents like splash mats or spill pillows catch liquids, and scooping up solids with a spatula beats brushing it with your sleeve. Never use your bare hands to clean up. Always suit up with fresh gloves and wipe down the area with plenty of soap and water. Dispose of contaminated materials right away—don’t let a paper towel full of residue sit around.
This isn’t a chemical you leave open for air to work on. Even if it doesn’t look volatile at room temperature, open bottles can pull in moisture or oxidize, ruining both your experiment and the next person’s. An amber glass bottle, tight lid, and a spot away from sunlight or heat sources help a lot. If your fridge or chemical cabinet comes with a lock, use it. Restricting access sounds restrictive, but if someone untrained stumbles across your bottle, that’s a headache you could avoid.
Tossing leftovers down the drain doesn’t cut it. Chemical waste goes in marked containers. I learned the hard way how even “routine” organics foul up plumbing or spike a building’s emissions. Ask your supervisor or safety officer if you don’t know which bin to use—better to be sheepish than to face an environmental fine.
Chewing pens or eating near your workspace brings chemicals closer to your mouth than you realize. Rushing or skipping over safety habits makes exposure more likely. Every year, I remind new lab members: a few extra seconds to put on glasses and gloves saves days lost to doctor visits or paperwork if things go wrong.
N-Boc-Pyrrole won’t jump out of the bottle, but it won’t do your safety work for you. The most protective lab environments don’t come from big budgets—they come from folks who look out for each other and don’t cut corners. Stick to these precautions, and you give yourself and your colleagues one less thing to worry about each day.
Anyone who’s spent time in a chemistry lab knows how the simple stuff, like picking out a bottle of reagent, can steer the whole direction of a project. N-Boc-Pyrrole isn’t some mystery compound—it's a workhorse in medicinal and organic synthesis. Its protective Boc group makes it valuable for those delicate reactions where you can’t let things run too wild.
Some labs run on a shoestring budget, others have deep pockets. If all you need is some quick proof-of-concept or you’re teaching a classroom exercise, technical or lower-purity N-Boc-Pyrrole goes a long way. It’s more affordable and still fits the bill. On the flip side, anyone making molecules for drug discovery or publishing serious work doesn’t settle for less than 97% or 98% purity. There’s no cutting corners. Too much junk in that bottle—unwanted side products, residual solvents, moisture—can ruin hours of work. High-purity grades also save you the pain of downstream purification. In my own experiments, a few tenths of a percent off spec once meant rerunning several columns—painful, slow, and not cheap.
Chemistry isn’t always about mixing big vats. Ten grams might last small-scale labs a whole year. Large process or scale-up operations chew through hundreds of grams, sometimes kilos. Buying too much opens you up to waste—chemicals break down over time, especially if hygroscopic or sensitive. Last year, my lab opened a half-used old bottle, only to find it had yellowed and clumped into uselessness. Too little, though, and you're paying repeat shipping, wrangling more invoices, and maybe facing shipment delays on tight deadlines. This awkward balancing act is where suppliers play a big role, offering multiple sizes: milligram vials for early stages and multi-gram jars for busier projects.
It’s not just about convenience. Flexible packaging cuts costs and limits waste, which actually lines up with mainstream lab sustainability goals now. Fewer oversized bottles headed for disposal means lower environmental risk. Plus, smaller quantities get around hazmat shipping headaches and can allow faster delivery.
Another piece of the puzzle is training—new chemists don’t always realize you can pick a custom size or purity. Labs that check in with their vendors or ask about specific formats actually save money and shelf space. Some companies will even portion certain specialty chemicals to match grant cycles or big collaboration projects. For example, a friend at a contract research lab told me how custom-sized bottles avoided a massive budget overrun and a mountain of leftover chemicals.
Strong networks with chemical suppliers make it easier to get what’s needed. A quick phone call sometimes unlocks new formats, even occasional bulk discount. Good suppliers ask what researchers plan to do with their product, guiding them toward a suitable purity and packaging setup. In a world where time in the lab feels almost as precious as funding, the right format and clean reagents can keep research moving forward, not sidetracked by unnecessary cleanups or worse, failed reactions. There’s no glory in slogging through three extra purification steps if a purer or fresher bottle could save the day.
| Names | |
| Preferred IUPAC name | tert-butyl 1H-pyrrole-1-carboxylate |
| Other names |
N-tert-Butoxycarbonylpyrrole 1-Boc-pyrrole tert-Butyl pyrrole-1-carboxylate Boc-pyrrole Pyrrole, N-(tert-butoxycarbonyl)- |
| Pronunciation | /ɛn-bɒk-paɪˈroʊl/ |
| Identifiers | |
| CAS Number | 145484-80-4 |
| 3D model (JSmol) | `3D model (JSmol)` string for **N-Boc-Pyrrole**: ``` CC(C)(C)OC(=O)N1C=CC=C1 ``` |
| Beilstein Reference | 136146 |
| ChEBI | CHEBI:131444 |
| ChEMBL | CHEMBL127470 |
| ChemSpider | 138073 |
| DrugBank | DB07744 |
| ECHA InfoCard | 01b2d9a7-2ca2-4bc9-93ae-80a4c5fcb180 |
| EC Number | EC 203-046-6 |
| Gmelin Reference | 787506 |
| KEGG | C11468 |
| MeSH | N-Boc-pyrroles |
| PubChem CID | 162064 |
| RTECS number | UQ2295000 |
| UNII | R43HL2T0E3 |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DTXSID70846173 |
| Properties | |
| Chemical formula | C9H13NO2 |
| Molar mass | 167.21 g/mol |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | 1.08 g/mL at 25 °C |
| Solubility in water | Insoluble |
| log P | 1.89 |
| Acidity (pKa) | 17.0 |
| Basicity (pKb) | 13.6 |
| Magnetic susceptibility (χ) | -33.41×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.498 |
| Viscosity | Viscosity: 0.897 cP (20°C) |
| Dipole moment | 3.10 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 299.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -424.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -5098.2 kJ·mol⁻¹ |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | P261, P305+P351+P338, P337+P313 |
| Flash point | 89 °C |
| Autoignition temperature | 88 °C |
| PEL (Permissible) | Not established |
| REL (Recommended) | 25°C |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
Pyrrole Boc anhydride N-Methylpyrrole N-Boc-piperidine N-Boc-indole |