Synthetic chemists once juggled plenty of risks and limits in crafting intermediates for pharmaceuticals and specialty chemicals. N-Boc-Piperazine became a go-to choice not by accident, but after years of chasing stable derivatives of piperazine that resist unwanted side reactions. Back in the days of early discovery, protecting groups largely meant improvisation and trial-and-error work. Boc, or tert-butoxycarbonyl, carried promise: it shielded nitrogen centers, took acidic conditions in stride, and peeled away cleanly when needed. Once the Boc group found footing in peptide chemistry, it didn’t take long for researchers to pair it with versatile heterocycles. N-Boc-Piperazine traces its roots to this era, offering just enough rigidity for selective chemistry, and the chemist’s knack for scavenging good ideas helped it spread through labs both academic and industrial.
Buy a vial of N-Boc-piperazine today, and you’ll usually receive a fine, white crystalline powder. It isn’t flashy or aromatic, but it brings consistency batch after batch. This chemical often appears anywhere a chemist wants a protected diamine. It enters the scene in drug discovery campaigns, advanced materials projects, life sciences, and even fine-tuned agrochemical programs. The product isn’t a building block for novices only—it’s found in the hands of major pharmaceutical developers, start-ups, and university labs eyeing a shortcut in tough synthetic sequences.
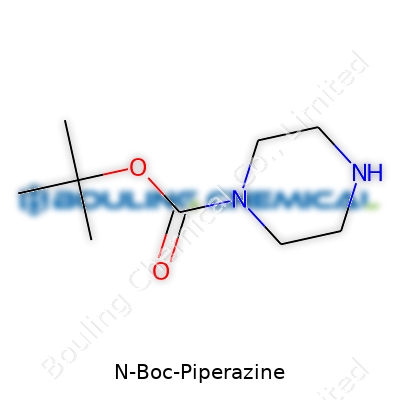
N-Boc-piperazine sports a molecular formula of C9H18N2O2, clocking in at a molecular weight near 186.25 g/mol. At room temperature, it forms a solid, melting in the range of 87°C to 91°C. It stays stable as long as it avoids strong acids, strong bases, and excessive heat. Its structure features a piperazine ring protected at one nitrogen by the Boc group, balancing chemical stability with future reactivity. The tert-butyl group on the carbamate adds some lipophilicity, making the compound handle organic solvents with ease. You won’t catch this compound catching fire easily either—the flash point sits comfortably far from regular operating conditions.
On arrival, the packaging usually announces purity above 97% by HPLC, which fits the bill for research and small-scale pharma work. Synonym lists include N-Tert-butoxycarbonyl-piperazine, 1-Boc-piperazine, and Boc-protected piperazine. Chemical structure diagrams grace data sheets alongside the familiar CAS number (57260-72-7). Safety labels point out the low acute toxicity but remind users to avoid swallowing, inhaling, or skin contact. In storage, a tight-sealed bottle in a cool, dry cabinet suffices. Material safety data sheets give details about potential dust inhalation, but with regular lab hygiene the risks remain tame compared to more reactive reagents.
The core synthetic approach hasn’t changed much in decades. Start with piperazine in excess, react it with di-tert-butyl dicarbonate and a base—usually triethylamine or sodium bicarbonate—in a suitable solvent like dichloromethane or acetonitrile. After the reaction, organic chemists lean on aqueous washes to knock out byproducts before evaporation and recrystallization polish up the final product. Sometimes, automated reactors or microfluidic chips enter the mix, helping to bump up yields or minimize solvent use as technology advances. What stands out is how robust these preparation steps have proven across both small-scale and bulk operations.
The Boc group works its magic by temporarily blocking the piperazine nitrogen. During multi-step syntheses, this allows selective reactions on just one nitrogen while the other remains untouchable. Tweak the protection to suit specific goals—swap Boc for Fmoc or Cbz groups if the process calls for it. When it’s time to move forward, deprotection is as simple as treating with acids like trifluoroacetic acid under controlled conditions. Once deprotected, piperazine derivatives are ready to link into larger pharmaceuticals or become cross-linkers in polymer projects, showing the utility of precision chemical blocking.
Pick your literature carefully, and you’ll find a parade of names: 1-(tert-Butoxycarbonyl)piperazine, Boc-piperazine, and N-Boc-hexahydropyrazine. The CAS registry lists it as 57260-72-7. Sigma-Aldrich, Thermo Fisher, TCI, and countless catalogues sell it under these aliases. Cross-checking these identifiers prevents expensive mix-ups during procurement or experimental planning. This cloud of synonyms keeps chemists careful but also ensures N-Boc-piperazine’s presence in reagent lists across continents.
Working with N-Boc-piperazine rarely stirs up dangerous fumes or runaway reactions. Simple rules apply: gloves on, goggles adjusted, and scales checked for spills. Spilled powder cleans up with a damp cloth, no drama. Inhalation or accidental ingestion calls for prompt action, though regular laboratory air changes minimize airborne dust. Most safety incidents tie back to poor storage or co-reacting with aggressive acids leading to hazardous byproducts. Technology’s push for better air filtration, more ergonomic packaging, and data-logging for chemical inventories all improve day-to-day operations, but smart habits anchored in real experience do most of the heavy lifting.
The story of Boc-piperazine travels through many research circles. Medicinal chemists love it for its ability to slip into combinatorial libraries for drug screening. Material scientists pick at it for new polymers and catalysts. In bioconjugation studies, it lets a single functional group attach to a protein or small molecule, cutting through unwanted cross-linking. Its mild conditions and high selectivity set the stage for multi-kilo pharmaceutical syntheses, and pricier derivatives tailor themselves for the ultimate target. This flexibility isn’t just theoretical—it shows up in marketed drugs, prototype sensors, and advanced coatings.
Researchers dig deeper into green chemistry by chipping away at the toxic solvents that once dominated Boc-protection. Microwaves, ultrasonication, and continuous-flow reactors edge out the old flask-and-fume hood model, offering cleaner product and faster cycles. Analytical teams layer on mass spectrometry and NMR spectroscopy to tease out even minor impurities. Cheminformatics software guides synthetic plans, cutting down the guesswork that once filled lab notebooks. Each new protocol builds a better path, sometimes raising the bar for sustainability, sometimes chasing efficiency, but always trying to get there just a little bit better than before.
The toxicological data on N-Boc-piperazine points to moderate safety for well-trained hands. Acute studies report low oral and dermal toxicity in rodents, and repeat-exposure trials suggest little bioaccumulation due to the molecule’s rapid hydrolysis in the body. Researchers rightly caution against letting it anywhere near food chains or waterways, since chronic, low-level exposures haven’t received the same scrutiny. At the bench, the substance doesn’t provoke the kind of reactions caused by strong alkylating agents or heavy metal catalysts. Still, as safety standards tighten, labs look to minimize waste and exposure through clever engineering, stricter inventory controls, and substituting more benign alternatives where chemistry allows.
N-Boc-piperazine’s place in advanced synthesis seems secure for now. Demand grows as medicinal chemistry explores new scaffolds or as companies scramble to meet regulatory demands for traceability and batch consistency. New approaches seek to swap out petrochemical sources for biobased feedstocks, promising greener supply chains. Computational chemistry and machine learning might streamline the hunt for even more effective protecting groups, but Boc has set a standard that future options have to match for ease of use and stability. Whatever shifts come, the practical lessons learned from decades with N-Boc-piperazine will shape choices from pharmaceutical giants down to the next generation of graduate students just starting out in the world of synthetic chemistry.
People often ask about the purity of N-Boc-piperazine, especially folks involved in pharmaceuticals, research chemistry, or custom synthesis. This chemical doesn’t exactly pop up on everyday shopping lists, so anyone searching out purity levels usually has something precise in mind. The lab-grade N-Boc-piperazine typically shows up with purities between 97% and 99%. A glance at product catalogs from Sigma-Aldrich or TCI shows those numbers cropping up again and again.
These percentages don’t only reflect what you’re likely to get from big suppliers. They speak to pressures from the industry. Drug development and research won’t tolerate mystery ingredients lurking in the mix. When I worked as a technician, a detail as small as a 1% impurity could spoil a week’s worth of experiments or confuse the results of an assay. For those setting up reactions where even minor impurities can foul things up, this difference between 97% and 99% can seem huge, even if it sounds trivial at a glance.
For a chemist, a bottle labeled as “>99%” purity suggests peace of mind. Yet in reality, nothing is ever truly 100%. Somewhere in each batch, a speck of water, a leftover solvent, or other chemical byproducts ride along. My own experience in analytical labs proved this: running an HPLC or NMR analysis often revealed something lurking in the baseline. Chemists learn to work with this, but producers know that once purity falls below 97%, most buyers start to lose trust — not just in the product, but in the supplier.
In largescale manufacturing or routine drug screening, labs rarely gamble on lower purity. One reason: impurities can react, break down, or interfere downstream. Synthetic pathways get stubborn when contaminants tag along. Labs depending on reliable reactions will pay extra for smaller containers marked “analytical grade” to guarantee every molecule counts. At my old lab, government inspections drilled into documentation for certified reference material and recorded every batch number, making high purity not merely a preference but a hard requirement.
There’s a flip side, though. Academic labs or budget-limited startups sometimes choose “technical grade” at 95% for pilot work. Saving money up front helps students get started, iterate, and learn. Industrial buyers who need tons for polymer or bulk synthesis may cut corners if minor impurities don't matter. Those decisions always rest on what happens next: Is the N-Boc-piperazine going toward an intermediate step, or does the final product end up in a clinical trial?
Sellers need to build trust with data. Certificates of analysis, consistent batch records, and transparency about testing methods help. If a vendor claims 99% purity, users will look for chromatograms and raw data to back it up. In my experience, some suppliers exaggerate, masking residual solvents or undetected byproducts. Labs need to stay sharp — confirming claims with in-house testing before letting chemistry move forward.
Raising the bar for purity demands better synthesis and cleaner processes. Green chemistry techniques cut down waste, automated purification tools trim faults in crystal growth, and sharper analytical equipment finds tinier contaminants. If institutions and buyers push suppliers to keep proof on hand, the industry steps forward. True progress doesn’t just show up in higher numbers on a label — it shows up in experiments that work, less waste, and deeper trust between buyers and sellers.
Anyone who has spent a few afternoons in a chemistry lab knows that chemicals rarely stay unchanged. Air gets in, moisture does its thing, and before long, things that once looked fine end up clumped or even ruined. N-Boc-Piperazine is no stranger to this. It’s not some drama queen of the chemical shelf, but the reality is, treating it with a little respect goes a long way.
N-Boc-Piperazine shows its best side when kept somewhere cool, dry, and shaded. Not “fridge packed with leftovers” cold, just a steady room temperature where the thermometer doesn’t jump up and down every hour. Put it on a shelf that doesn’t get much sun. Direct sunlight is a quick way to break it down—those UV rays are sneaky, and over time, they do a number on the compound. The same goes for humidity. Leaving the jar open after you scoop some out brings moisture in, making the compound cake together, or worse, degrade.
A tight cap is not just about avoiding spills. Air carries more than just oxygen; there’s moisture and all sorts of dust that aren’t friends with N-Boc-Piperazine. Most lab-grade suppliers ship this stuff in amber bottles for a reason. Light protection counts for a lot, and a solid screw cap keeps out what shouldn’t get in. It’s always tempting to leave jars cracked open during a busy synthesis, but every shortcut adds up. Even a bit of ambient moisture creeping in will clump things or invite slow reactions you don’t see coming.
Folks handling N-Boc-Piperazine usually work on multi-step syntheses. Impurities that sneak in through sloppy storage may not hurt things right away, but give it some time, or raise the stakes with a more sensitive reaction, and problems start rolling. It’s easy to overlook small changes—maybe a slightly off color or a bit more stickiness. After a while, crude yield drops or a key intermediate doesn’t crystallize the way it should. I’ve watched more than a few people chase their tails looking for mystery causes, when the problem started at the storage shelf months back.
Keep things simple. Use small bottles if you can. That way, you open only what you need, leaving the main supply untouched by constant air and moisture. Label each with the date you opened them. It sounds a bit overboard, but tracking age helps weed out old stock before trouble starts.
Desiccators look fussy, but they’re a lifesaver for chemicals like this. Pop the bottle inside with a fresh silica pack and you cut down on humidity worries. If the storage room gets humid in summer, a basic dehumidifier makes a surprising difference. Skip high shelves near lab lights since heat rises—stick with eye-level racks, away from windows and radiators.
If you catch a bottle that’s clumped, yellowed, or oddly smelly, your best bet is to toss it. Don’t try to “fix” compromised stock with tricks like re-drying. Risking a whole synthesis for a few grams just isn’t worth it. In every lab I’ve worked, minor losses pale in comparison to failed reactions or weeks lost troubleshooting.
N-Boc-Piperazine doesn’t demand heroics. A little routine—proper caps, dry spaces, keeping light at bay—protects your time and your work. Chemistry often feels complicated enough. Building good storage habits saves you energy for the problems that really matter, and that’s something every chemist I know can appreciate.
Folks in chemistry circles know N-Boc-Piperazine well. If you’ve spent any time around a pharma lab or a grad student with too many unlabeled vials on their bench, you’ve probably run into it. Every chemist chasing new molecules for medicine appreciates a good protective group, and that’s N-Boc-Piperazine’s main gig. By wearing that Boc group like a raincoat, piperazine keeps one end protected, so the rest can jump into reactions without fuss.
It’s a simple trick, but it changes the game. Chemistry can get messy — react one side, and the other side wakes up cranky, ruins your yield, and chews through your budget. Pipe in a Boc group, and one half stays quiet, letting you build a new molecule in fewer steps. In drug discovery, this saves weeks. In industry, it can save millions. I’ve talked with researchers who swear it made their graduate projects actually work after months of failed syntheses.
Most folks outside labs never hear about this stuff, though drugs that start as N-Boc-Piperazine wind up in medicine cabinets. Drug designers rely on piperazine parts to build up painkillers, antibiotics, antiviral pills, or psychiatric meds. Take a look at streamline processes for synthesizing antihistamines, antipsychotics, or even cancer-fighting compounds. The presence of a Boc protecting group makes those complicated chemical recipes a bit more manageable. That means less waste and cleaner ways to produce trial batches for pharma companies.
I worked for a summer with an organic chemist who spent half his life on late nights—troubleshooting reactions that jumped off the rails. He said the highlight of his career was figuring out how to toss in a Boc-piperazine step and finally walk home before midnight. That story sounds small, except that small shifts like these, repeated across thousands of labs, speed up the whole drug development pipeline and make new cures possible.
N-Boc-Piperazine also finds a home in material science. Some smart folks in polymer research use it as a starting point for building blocks in high-tech coatings or electronic materials. It helps create chainlike structures, stitch new materials together, or tweak surface properties. Not glamorous, but the foundation for flashy new materials that show up in batteries, sensors, or even medical devices.
I spent a few months trying to design molecules for a simple, self-healing plastic in college. We hit wall after wall until a fresh delivery of N-Boc-Piperazine gave us a new route. It’s not a silver bullet, but it opens up avenues for chemical creativity. Being able to block parts of a molecule, build what you want, then pull off the protection at the perfect moment gives scientists more control over their creations.
Using chemicals like N-Boc-Piperazine comes with the usual setup: costs, safety, waste, and practical hiccups. If you want greener chemistry, look for ways to limit or recycle the Boc group, tweak production methods, or invent more environmentally friendly protection that still gets the job done without all the cleanup. Teams working on “click chemistry” and microwave reactors report some progress. Labs with better waste management and cleaner solvents can help too. The trick lies in moving from good chemistry to chemistry that won’t leave us with a pile of problems to solve down the line.
Ask anyone who buys groceries or supplies in bulk—packaging size isn’t just a technical detail. It shapes what people buy, how much waste ends up in the trash, and even how much money stays in a family’s pocket. For years, I’ve found that bulk options usually win for value, especially at stores like Costco or Sam’s Club. The up-front price seems higher, but breaking it down per serving or per ounce, families benefit. On the other end, single-serve packets—the kind tucked into a lunchbox—keep food fresh but cost a whole lot more in the long run and stuff our landfills with wrappers.
Small business owners I’ve spoken with point to packaging size as a make-or-break factor. Take a neighborhood café. A five-pound sack of flour makes sense for a low-volume bakery. For a diner serving hundreds each day, those sacks slow the kitchen down. Twenty-five or fifty-pound bags cost less per pound, cut down on storage trips, and usually last the week. If the café orders only single-use packs meant for home cooks, that kitchen’s drowning in trash bags by noon.
Then there’s the other side of choice. Let’s say a person lives alone and has limited kitchen space. He buys a massive container of peanut butter thinking it’s cheaper. But it goes bad before he finishes. Smaller jars save him from wasting food and prevent spoiled surprises lurking in the fridge.
Food waste groups keep pounding the same drum: oversized packages, especially perishables, end up half-used and tossed. Research from the USDA has shown that households buy in bulk to save, but often lose the savings by dumping food that spoiled before being used. Smaller bottles, cartons, and pouches cut down on waste. At the same time, more little packages spell more plastic and cardboard, so the trash piles up—one problem replaced by another.
Smart retailers have tried to bridge both sides—offering products in a range of sizes. Think about stores that let you scoop grains or nuts into your own reusable containers, paying by weight. This idea isn’t new; it’s how older generations bought most staples. Less packaging, less waste, just what you need. For companies without bulk bins, offering mid-size options—say, half the bulk container—helps single folks and families both waste less and save money.
Laws in some countries have started to nudge manufacturers toward using recycled or recyclable packaging. Some food companies even collect back empty containers and give a deposit discount—a little throwback to the old glass milk bottle days. The move grows slowly, but it gives hope for cutting down on both wasted food and packaging trash as a double win.
For people shopping for products, reading the price per unit right there on the store shelf saves money. Choosing what matches the household’s real needs beats falling for the “bigger is better” myth. Also, asking local stores to offer a few more packaging sizes—even organizing a petition—gets the message to decision makers. I’ve seen this work in small towns where shoppers wanted smaller juice bottles for schoolkids. The store responded, and waste from half-finished bottles dropped fast.
Packaging size shapes more than a shopping cart. It touches our wallets, the planet, and even what we’re able to cook for dinner tonight. Your choice—and your voice—matter in how those products arrive on the shelf.
Ask anyone who spends time around synthetic chemistry and they’ll know about N-Boc-piperazine. This stuff shows up all over the place, usually as an intermediate in pharmaceutical research. I’ve seen it firsthand during stints in academic and contract research labs. People sometimes act like “protecting groups” like Boc magically make everything safer, but that’s not always the case.
Grab the safety datasheet, and it tells a simple story. N-Boc-piperazine has a reputation as an irritant. Get the powder on your skin, eyes, or inhale a little dust and you’re likely to end up with discomfort—sometimes a nasty rash or red, burning eyes. On paper, it doesn’t have the deadliest profile out there. Still, regular exposure without protection just isn’t worth it. One person in my old lab decided to skip gloves once, and it only took thirty seconds before his fingers started reacting.
Gloves, goggles, and working under a decent fume hood aren’t overkill. People get lazy under pressure, but taking shortcuts means you trade a couple of minutes now for hours of discomfort later. Breathing in powders never ends well, especially with organic chemicals. A decent dust mask stops the microscopic stuff from finding its way into your nose—or worse, your lungs.
Like a lot of organic compounds, N-Boc-piperazine lives longest in a cool, dry spot with a tight lid. Water and air slowly tear down the Boc group. Once you open a bottle, moisture from the air starts its work. I’ve seen some folks toss a bottle on a bench for weeks and wonder why it turns clumpy, mushy, and hard to weigh. Keeping the lid screwed tight and stashing it in a desiccator keeps things flowing and predictable.
Label everything clearly. In a busy work environment, someone else might grab the wrong bottle. Trust me, confusion like that leads to lost samples, ruined reactions, or someone using hazardous chemicals without realizing it.
Small spills happen—think dust scattered on the scale or a tiny pile knocked over. Sweep it up carefully with a disposable towel or wet wipes, not your bare hands. Scrubbing at it dry just stirs the powder up, making it easier for everyone to breathe it in. For bigger messes, wet a towel, clean it up, and toss it in the chemical waste, not the regular trash.
Many chemists spend years around these kinds of reagents. Even if one contact doesn’t harm you, repeating these slip-ups day in, day out builds up. There’s still a lot we don’t know about chronic exposure. Playing it safe avoids waking up a decade from now with allergies or skin sensitivities that keep you out of the lab—or worse.
I’ve always pushed for a safety culture where people watch out for each other. Putting tools—like gloves and goggles—out in the open makes it more likely everyone will use them. Nobody wants to be the reason someone else gets hurt, or bring something home on their clothing to their family.
Handling N-Boc-piperazine safely isn’t just common sense; it’s practical and respectful to yourself and your coworkers. Careful storage, good labeling, and routine cleanup keep the workplace safer and more efficient. Getting in the habit now means you’re ready for every other chemical down the line, not just this one.
| Names | |
| Preferred IUPAC name | tert-butyl 4-piperazinecarboxylate |
| Other names |
1-Boc-piperazine tert-Butyl piperazine-1-carboxylate N-tert-Butoxycarbonyl piperazine Piperazine, 1-(tert-butoxycarbonyl)- Boc-Pip |
| Pronunciation | /ɛn-bɒk-paɪˈpɛrəziːn/ |
| Identifiers | |
| CAS Number | 57260-72-7 |
| 3D model (JSmol) | `3D structure; JSmol string:` `CC(C)(=O)OC1CNCCN1` |
| Beilstein Reference | 3011570 |
| ChEBI | CHEBI:131769 |
| ChEMBL | CHEMBL418115 |
| ChemSpider | 157354 |
| DrugBank | DB14147 |
| ECHA InfoCard | 07b1c7dc-9e4a-4eb1-9d6d-199ebdbba2d7 |
| EC Number | 6669-10-3 |
| Gmelin Reference | 85735 |
| KEGG | C14147 |
| MeSH | D010999 |
| PubChem CID | 119895 |
| RTECS number | TZ1940000 |
| UNII | U40C8K85DS |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DTXSID2040721 |
| Properties | |
| Chemical formula | C9H18N2O2 |
| Molar mass | 217.29 g/mol |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | 1.08 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | -0.22 |
| Acidity (pKa) | pKa ≈ 9.8 |
| Basicity (pKb) | 3.8 |
| Magnetic susceptibility (χ) | -62.42 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.486 |
| Viscosity | Viscous oil |
| Dipole moment | 3.67 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 270.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -555.7 kJ/mol |
| Pharmacology | |
| ATC code | |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS06, GHS08 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | P264, P280, P302+P352, P305+P351+P338, P332+P313, P337+P313, P362+P364 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 100.2 °C |
| LD50 (median dose) | LD50 (median dose) of N-Boc-Piperazine: "LD50 (rat, oral) > 2000 mg/kg |
| NIOSH | Not Listed |
| REL (Recommended) | 25 - 30°C |
| IDLH (Immediate danger) | NIOSH has not established an IDLH value for N-Boc-Piperazine. |
| Related compounds | |
| Related compounds |
Piperazine Boc-Anhydride N-Methylpiperazine N-Boc-Pyrrolidine N-Boc-Piperidine N-Acetylpiperazine N-Boc-Morpholine N-Tosylpiperazine Boc-protected amines |