Chemists started paying more attention to the imidazole ring just over a century ago, right around the time when pharmaceuticals were beginning to leave folk wisdom behind and step into the lab. N-Benzylimidazole, with that benzyl group attached to the nitrogen, grew out of early synthetic work focused on tweaking the core heterocycle for new effects. The first documented preparations reached the published world back in the days of classic German synthesis, when researchers explored the balance between basicity and reactivity in these rings. Decade by decade, applications grew—pharmaceutics, material science, and organic synthesis all found a place for this structure. The mid-20th century saw researchers ramp up production routes, leaning on both academic and industrial research to unlock new routes and scale up yield. Now, N-Benzylimidazole lands on the bench not just as a scientific curiosity, but as a practical toolkit staple that owes its place to this layered, evolutionary history.
Anyone who’s handled N-Benzylimidazole in the lab knows it brings plenty of flexibility to the table. Its molecular structure—imidazole paired with a benzyl group—offers a neat balance between stability and reactivity. This compound shows up as a white to off-white crystalline solid, a detail that keeps handling straightforward for most standard procedures. More than many, it leans into its role as an intermediate; whether someone’s working up a new API candidate or adjusting a catalyst’s behavior, N-Benzylimidazole manages to fit into projects that favor modular building blocks.
Looking at the numbers, it’s clear why N-Benzylimidazole stands out. The molecular formula C10H10N2, and a molar mass right around 158.2 g/mol, keep calculations tidy, especially during scale-up. Its melting point hovers close to 48–50 °C, so it doesn’t demand exotic handling or expensive containment. The compound dissolves smoothly in common organic solvents like methanol, dichloromethane, or even DMF, which expands options for reaction conditions. N-Benzylimidazole’s basicity surpasses that of unsubstituted imidazole, thanks mostly to the electron-donating benzyl group. Up in daily work, it resists air and moisture far better than some of its more hydrophilic siblings, letting chemists store it on the shelf without sweating every humidity swing.
Most reputable suppliers package N-Benzylimidazole with purity clearly labeled, commonly above 98% for research-grade lots—enough for just about any bench application. Look for density figures (about 1.1 g/cm³ at 20°C), Melting point (usually in the 48-50°C bracket), and details like CAS Number (616-39-7) front and center on every label. Safety information sits next to handling guidelines, including recommended storage temperatures (dry, below 25°C), hazard pictograms, and a reminder that gloves, goggles, and adequate ventilation keep the risks in check. Some lots describe trace impurities or isomeric content; researchers running sensitive reactions check that spec sheet before risking a whole batch.
Take a quick look at modern literature and most routes to N-Benzylimidazole lean on the alkylation of imidazole with benzyl halides, especially benzyl chloride or benzyl bromide. The base—often potassium carbonate or sodium hydroxide—deprotonates the imidazole and gets the reaction moving. Stirring in an organic solvent like acetone or DMF, chemists drive the alkylation to completion, then chase the product through workup, filtration, and recrystallization. Some labs use phase-transfer catalysis to smooth out bottlenecks, while others run microwave-assisted variations for speed. No matter the technique, yields consistently push north of 80% with decent optimization, a nod to just how well-developed the protocol has become.
N-Benzylimidazole steps up as a nucleophilic participant in a spread of transformations. The benzyl group, while blocking N1 from further substitution, turns out to be a strategic switch. Removal under hydrogenolysis returns the parent imidazole, letting researchers play with protecting group strategies. Electrophilic aromatic substitution is less dramatic on the imidazole ring itself, but chemists have managed selective halogenations and nitrations at the C5 position. Coupling reactions—think Suzuki or Heck—can proceed smoothly when someone swaps the benzyl group for a more functional leaving group. In coordination chemistry, that lone pair on the nitrogen finds a happy partner in transition metal complexes, leading to catalytic systems with tweaked reactivity. These modifications breathe new life into the core imidazole scaffold, setting up libraries for drug discovery or material science.
A quick scan through catalogues or databases reveals a handful of alternate names: N-Benzyl-1H-imidazole, 1-Benzylimidazole, and 616-39-7 as the CAS number. Commercial labels sometimes use abbreviated forms like BzIm, especially when sold as part of a compound set. This jumble of synonyms doesn’t always make record-keeping easier; anyone managing a lab inventory needs sharp attention to avoid doubling up on equivalent stock. For procurement, spelling out the full IUPAC name makes conversations smoother between buyers and suppliers, especially across different regulatory regions.
N-Benzylimidazole rates as a low-to-moderate hazard, mostly on account of its potential as a skin and eye irritant. Standard PPE—nitrile gloves, splash goggles, and fume hoods—covers the risks. Labs store it sealed, away from strong oxidizers or moisture. Material Safety Data Sheets highlight a flash point above 150°C and a low volatility, ruling out most runaway hazards under typical use. Spills call for dry absorption and careful cleanup; users with asthma take care, since even mild bioactivity can provoke reactions in sensitive workers. Strict labeling—clear hazard pictograms and R/S phrases—make sure no risks sneak past busy hands on a crowded bench.
Pharmaceutical synthesis grabs big headlines, as N-Benzylimidazole acts as a key intermediate for antifungal agents, muscle relaxants, and investigational drug scaffolds. In coordination chemistry, its unique binding sites give researchers new ways to tailor metal complex behavior—essential for designing catalysts or sensor systems. Polymer chemistry, too, has discovered its value, folding this molecule into functionalized materials for coatings, sensors, and responsive membranes. Analytical labs use it as a reference, standard, or derivatization agent, extending the reach of routine techniques. On a different front, it even steps in as a corrosion inhibitor in select industrial fluids, proving that careful formulation unlocks performance beyond just pharmaceutical ambitions.
Labs continue to experiment with new synthetic routes and modifications to the imidazole ring, hunting for improved biological activity, better environmental performance, or increased selectivity. Structure-activity relationship (SAR) studies rely on libraries built around N-Benzylimidazole and its analogues to sharpen the focus on lead compound optimization. Teams working at the interface of organic and inorganic chemistry report fresh coordination complexes that stretch known reactivity. High-throughput reaction platforms use it as a base structure, slotting in new substituents with robotic precision. The body of patents referencing N-Benzylimidazole keeps expanding, proof that inventors see unexplored spaces in both end-use and process chemistry. In green chemistry, process engineers are working to swap out hazardous solvents or improve atom efficiency, showing a growing shift toward sustainability, even in well-worn territory.
The toxicological data for N-Benzylimidazole doesn’t flag any dramatic hazards, but responsible labs keep a close eye. Acute oral toxicity in rodents lands in the low-millimole-per-kilogram range, putting it well behind truly hazardous reagents. Repeat exposure studies over days or weeks have found no mutagenic tendencies or evidence of chronic harm at occupational doses. That said, any compound capable of crossing biological membranes and binding proteins deserves respect. Waste streams containing this material must avoid open disposal, following standard hazardous organics protocols to keep downstream waterways and soils clean. For pharmaceutical derivatives, in-depth metabolic and environmental impact studies continue to fill important gaps.
With the rising demand for tailor-made catalysts and advanced pharmaceutical intermediates, N-Benzylimidazole is lining up for more significant roles across chemical industries. Currently, green chemistry improvements target the legacy alkylation routes, aiming for reduced waste and fewer harsh reagents. Machine learning and automated screening platforms are fast-tracking analog design, and this compound serves as a workhorse for iterative, data-driven research. Biomedical teams may soon uncover new uses, as imidazole scaffolds have a habit of popping up in unexpected therapeutic leads. Environmental safety and regulatory expectations keep tightening, so companies manufacturing or processing N-Benzylimidazole are under pressure to sharpen supply chain stewardship and invest in safe disposal. Every time the chemical toolbox expands, the chances for a well-understood, adaptable molecule like N-Benzylimidazole expand along with it—not just as a stepping stone, but as a tested foundation for creative science.
There's a big fuss in lab circles about the purity of compounds like N-Benzylimidazole. In practice, those numbers on the bottle mean more than scientists often admit. I’ve seen colleagues treat purity percentages as an afterthought, only to have their experiments wobble and stall when they try to reproduce their results. This isn’t just about perfectionism or chasing decimals. Purity levels sneak into the heart of every reaction, coloring the outcome, making or breaking an entire synthesis.
Lab suppliers usually quote purity using percentages, something like 98% or 99%. On paper, that seems close enough to perfect. The difference those stray percentages make can feel trivial until a side reaction or stray contaminant rears its head. I’ve worked on syntheses where even half a percent of impurity led to byproducts piling up, ruining yields and, once, fouling a whole column that took weeks to clean.
N-Benzylimidazole comes from reaction paths that can introduce a real rogues’ gallery of side products: leftover starting materials, solvents clinging stubbornly to the product, trace metals slipped in from old glassware, and decomposition products that sneak in during long storage. It’s easy to forget how many fingerprints show up, from poor-quality recirculated solvents to dust from careless handling. Each one chips away at the advertised purity.
Working with impure N-Benzylimidazole isn’t just about pride or accuracy. In some pharmaceutical work I’ve followed, contamination can seriously muddy toxicology results. Med chemists risk wasting thousands of dollars on animal studies if test compounds contain mystery ingredients. I’ve seen regulatory agencies toss out months of research because spectral data didn’t back up purity claims.
Even outside high-stakes pharma, low purity crops up as extra spots on a TLC plate, surprise peaks in an NMR, or ghost signals in mass spec results. These can make troubleshooting agonizing and eat up time budgets faster than anyone likes to admit. Now, batch-to-batch variation sparked by changing suppliers or lots only makes things trickier.
Don’t take label claims for granted. A lot of chemists run their own NMR or HPLC after opening a new bottle, just to be sure. Sometimes, the numbers line up; sometimes, surprises pop up, and not the good kind. A supplier’s reputation helps, but nothing beats checking for yourself, especially if your project budget hangs in the balance.
Purify it yourself? That’s an option I’ve had to fall back on more than once. Recrystallization, distillation under reduced pressure, or even column chromatography all work if time allows. It’s not glamorous, and results can vary, but it’s better than blaming another failed run on a supplier who cut corners.
For folks without heavy-duty analytical tools, old-school tricks—washing crystals, careful drying, or running a melting point test—still give clues. The cleaner your input, the fewer ugly variables creep in downstream, no matter what the reaction goals are.
Those numbers reflect hours of work, hidden headaches, and trust built between researchers. Anyone serious about using N-Benzylimidazole, especially at scale, learns to sweat the small stuff, measure twice, and save the frustration for the stuff outside their control. Few shortcuts pay off for long.
Most people don’t wake up worrying about N-Benzylimidazole. Yet, if you work in a lab or around chemicals, storage goes right up there with safety goggles and gloves. This compound doesn’t ask for something exotic but regular attention makes all the difference between, "I can use this," and, "We need a new bottle."
Years back, I watched a technician toss chemicals onto a cluttered shelf, next to a window that baked in the afternoon sun. It didn’t take long before the bottles collected dust—some contents cracked, others changed color completely. That sweat-on-the-brow moment reminded me: a little planning up front saves money, headaches, and sometimes a report to safety.
N-Benzylimidazole asks for a reasonable routine: stow in a cool, dry place, out of sunlight, far from moisture. This is for a reason—temperature swings speed up chemical break-down, and damp air can introduce unwanted reactions. If it clumps, yellow-browns, or gives off unfamiliar smells, that’s nature’s way of saying it’s not the compound you started with.
Fancy fridge tech isn’t needed. A basic cabinet with reliable airflow does the trick as long as it’s away from radiators or heaters. Aim for storage around room temperature (15-25°C), not the freezer or the greenhouse. Humidity ranks just as high: dry means less chance for caking, sticking, or mysterious liquids at the bottom of the bottle. This also means avoiding sinks or bathrooms—places where humidity hangs in the air.
Direct sun turns some chemicals into experiment zones. I’ve seen labels bleach out so badly, nobody could read them. That’s not a small thing—relabeling introduces mix-ups, and no one wants a guessing game. Dark, closed cabinets shield not just the bottle but its label, instructions, and your sanity.
I once found a half-open chemical container wedged on its side and asked myself how long it had been like that. Exposure like this means fumes escape, air moves in, and suddenly chemical purity is only theoretical. N-Benzylimidazole likes bottles tightly closed and upright. Make sure the cap’s on all the way—hand-tight is good, but don’t crank it down until plastic or threads strip.
A shelf stocked like a game of chemical Tetris makes for trouble. Some compounds—acids, strong oxidizers—react violently even from vapors alone. Keeping N-Benzylimidazole separated from those is more than a suggestion, it’s a line between routine day and disaster. Use clear labeling, group by compatibility, and keep a quick reference chart nearby. That habit has rescued me from close calls more than once.
Even the best conditions don’t stop time. Stale chemicals surprise no one who checks their registry often. Mark open dates; clear expired bottles without delay. Finished bottles go for chemical waste, not the regular bin. If there’s a spill, grab gloves and get to it; chalky streaks lead to questions about safety long after the mess dries up.
Storing N-Benzylimidazole safely doesn’t demand fancy tools, just steady habits. Label clearly, stay organized, check pairs and avoid overcrowding. A clean storage space signals respect for your workspace and future experiments. That’s the real fix—taking daily responsibility to heart, so you never have to wonder what’s really inside the bottle next time you need it.
Talk to any chemist who’s spent time in a lab, and chances are they’ve bumped into N-Benzylimidazole at some point. The name sounds technical, but it’s a fairly simple structure at its core—a benzyl group attached to an imidazole ring. This combo turns out to be a solid workhorse in plenty of settings, even if you don’t hear about it as often as other, flashier chemicals.
I once worked alongside a team testing potential drug molecules. N-Benzylimidazole popped up time and again as part of the chemical toolbox. It forms the backbone for several pharmaceutical compounds, especially ones looking to tweak biological pathways for diseases. Researchers rely on it during synthesis, sometimes to add stability, sometimes to get just the right molecule shape. Its presence can make drugs easier to produce or less likely to break down too quickly inside the body. The race for new antibiotics, antifungals, or cancer drugs often runs through synthesis routes featuring imidazole-based structures.
Years ago, I watched as a batch reactor hummed along, and N-Benzylimidazole quietly did its job as a ligand. Ligands bind with metals, and suddenly chemists can run reactions that just wouldn’t happen otherwise. Some industries use this effect to push along chemical transformations that make fragrances, plastics, or coatings. I remember trying to separate metal ions in a waste stream—adding a compound based on N-Benzylimidazole kept the process both selective and efficient. It sped things up, saved resources, and helped reduce what would otherwise be chemical trash.
Corrosion eats up millions in damages every year. My uncle, an engineer, pointed out that N-Benzylimidazole can fight rust and tarnish on copper and certain alloys. Plant managers might coat pipes or wiring with solutions containing imidazole derivatives. This extra protection helps electrical systems keep running, which means fewer headaches for workers hunting down shorts caused by corrosion. Anyone overseeing maintenance for big machinery appreciates any product that cuts down on repair costs, and N-Benzylimidazole gets the nod in a few of these anti-corrosion paints and treatments.
Take a walk down the cleaning aisle of any store, and you’ll find ingredients derived from imidazoles. Builders and formulating chemists reach for N-Benzylimidazole as a helper—the sort of thing that doesn’t grab headlines but quietly keeps cleaning pads, liquid solutions, and stain removers working the way they should. The compound can also slip into flavor and fragrance production, giving perfumers a little bit more creative freedom. Regulatory folks keep an eye on it, so you won’t find it tossed around carelessly. The recipes get balanced, and the end goods usually pass safety checks.
N-Benzylimidazole clearly plays a practical role behind the scenes. While not every use is risk-free, responsible sourcing and smart process management keep mishaps rare. I’ve seen labs where a slip-up meant halting the project to review protocols—nobody wants surprises from chemicals someone overlooked. Strong labeling and routine checks keep the workplace, and end users, out of harm’s way. For folks in science, engineering, or just patching up a leaky pipe, this one small molecule adds real value, often without a spotlight.
N-Benzylimidazole acts as a key ingredient in chemical manufacturing, especially for people working in research labs or industrial production lines. There’s more to this substance than just its molecular makeup. In practice, the question of how this compound reaches users—what sizes it comes in, how much you can order at a time—can really shape how projects run.
Not everyone needs—or wants—a fifty-kilogram drum sitting on their workbench. For graduate students, a small glass bottle holds plenty. Lab technicians working on synthetic pathways do not want the headaches that come from wrestling with outsized packages. On the other hand, I’ve run into situations where buying bulk offered a significant discount and less waste, especially for larger chemical operations or manufacturing jobs that chew through materials by the week. Manufacturers usually offer various options, ranging from compact bottles for testing up to drums for high-volume needs.
Storing chemicals safely should always be in the conversation. Larger containers make sense for big companies with proper facilities, but for someone running reliability studies in a shared university environment, smaller packages make handling less risky. I recall a few close calls in cramped spaces—ordering a single, manageable container makes a real difference for safety. It’s not just about fitting it on the shelf. Spill response, exposure risks, and even shelf life change with package size. The more that’s opened, the greater the chance for accidents. Breaking up orders into several smaller bottles can keep things safer, especially if multiple teams use the inventory.
It’s tough justifying a bulk purchase if a project budget stays tight, or if the material spoils before the next round of funding. Most suppliers recognize this. From my own experience working with lean research teams, there’s value in only buying what you’ll actually use. More packaging sizes mean more flexibility. Minimizing waste becomes more than an environmental fad; it’s common sense. No one wants half-empty drums of expired chemicals headed for disposal. Choosing smaller sizes can help organizations save both money and storage space.
Supply chains have grown unpredictable over the past few years. Getting the right amount of N-Benzylimidazole sometimes comes down to whether the supplier has it in stock in the size needed. Delays or shortages push people to adjust. In response, chemical suppliers who keep an assortment of package sizes give their customers an edge—they’re less likely to stall projects. Research teams under deadline would rather grab two 1-kg bottles than wait weeks for a 5-kg container. I’ve seen projects grind to a halt over a single missing shipment. Flexibility in sizes helps everyone keep experiments and production lines rolling.
Ordering chemicals shouldn’t feel like a guessing game. Move beyond the one-size-fits-all mindset and the process becomes smoother. Often, a handful of standard sizes can cover most user needs. Firms who listen to feedback and keep common options on hand earn trust fast. For anyone managing a chemical inventory—whether in a university stockroom or a commercial plant—simple, dependable choices make procurement much easier.
Offering N-Benzylimidazole in a variety of packages hardly seems glamorous. Yet, this decision ripples through science and industry. Everyone from researchers to procurement officers stands to gain. Thoughtful size options lower risks, cut costs, and make supply chains more resilient.
Walking through the world of chemicals, I’ve learned that the language of science can look cold: just a set of digits and some cryptic formulas. N-Benzylimidazole’s CAS number—hint, it’s 1212-99-3—and its molecular formula—C10H10N2—show up at the top of safety sheets and shippers’ manifests, but there’s far more under the surface than those strings of characters.
People in labs use CAS numbers like names in a small town. Each CAS (Chemical Abstracts Service) number points to one substance. No mix-ups, no “almosts.” That precision removes guesswork, keeps shipments straight, and limits risk. I once watched a colleague search for a reagent and grab one bottle, then stop and check the CAS. That habit has saved more than a few experiments. The same goes for the molecular formula. C10H10N2 isn’t just a string for paperwork—it spells out ten carbons, ten hydrogens, and two nitrogens, baked into one stable ring foundation. For chemists, that matters every time they plan a reaction or check purity.
N-Benzylimidazole sticks in my mind because I saw it pop up as a building block in the pharmaceutical industry. Its imidazole ring carries special properties, often used to build drugs, corrosion inhibitors, or catalysts. Each time I’ve seen it used, nobody cared what the bottle looked like or how much it cost; the discussion started with its identity. Without that CAS number, mistakes could have moved from annoying to dangerous in a hurry.
Even small research labs lean heavily on accurate compound IDs. Someone eager to move quickly, chasing a deadline, spots “N-benzylimidazole” in the catalog. Another analog could sit right next to it, nearly the same name but a small difference in structure. Only that unique CAS number puts the brakes on a wrong order, a wasted week, or a ruined experiment.
I’ve met scientists who keep CAS numbers on sticky notes by their desks. Others have them memorized for the handful of chemicals they use daily. It isn’t obsession; it’s about safety and getting the work right. I watched a mishap unfold once just because an assistant skipped checking the CAS number. Fortunately, it only cost a day rather than a hazard, but that lesson stuck.
Clear labeling, strong training for newcomers, and double-checking before opening a bottle—these simple habits beat fancy software for practical safety. Even if automation grows, human eyes and experience make up the last line of defense. I’ve seen how sharing real-world stories about mix-ups and teaching with hands-on examples helps drive home the importance of those numbers and formulas, far more than a dry read-through of a datasheet.
Mistakes don’t disappear because a lab runs smoothly. Stress and turnover bring slips. Ongoing education, sharing near-miss stories, and making the risks real—these steps do more than posters ever will. Adding QR codes that link straight to reliable databases right on chemical bottles could shrink errors further. People learn quickly when the consequences become clear and the resources directly support their work.
That CAS number—1212-99-3—and that formula—C10H10N2—mark N-Benzylimidazole as unique, both on paper and in practical lab life. Pairing hard data with lived experience tightens safety, builds skill, and shows why details matter in the real world.
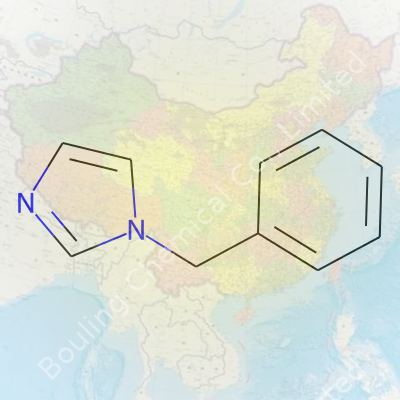
| Names | |
| Preferred IUPAC name | 1-benzyl-1H-imidazole |
| Other names |
1-Benzylimidazole N-Benzyl-1H-imidazole |
| Pronunciation | /ɛn ˈbɛn.zɪl ɪˌmɪd.əˈzoʊl/ |
| Identifiers | |
| CAS Number | 933-67-5 |
| Beilstein Reference | 1205951 |
| ChEBI | CHEBI:35684 |
| ChEMBL | CHEMBL107021 |
| ChemSpider | 74310 |
| DrugBank | DB08347 |
| ECHA InfoCard | 69b392317b61-44b0-9cab-581795e10164 |
| EC Number | 612-335-3 |
| Gmelin Reference | 7781 |
| KEGG | C12102 |
| MeSH | D017965 |
| PubChem CID | 85516 |
| RTECS number | NL8580000 |
| UNII | A4K642S2IX |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DTXSID5022305 |
| Properties | |
| Chemical formula | C10H10N2 |
| Molar mass | 194.25 g/mol |
| Appearance | White to light yellow crystalline powder |
| Odor | faint amine odor |
| Density | 1.08 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 1.49 |
| Vapor pressure | 0.00147 mmHg at 25°C |
| Acidity (pKa) | 6.9 |
| Basicity (pKb) | 7.55 |
| Magnetic susceptibility (χ) | -66.6·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.614 |
| Viscosity | 26.6 mPa·s (25 °C) |
| Dipole moment | 3.73 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 192.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -4.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4410 kJ/mol |
| Pharmacology | |
| ATC code | N03AX17 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | Precautionary statements: P261, P280, P305+P351+P338, P337+P313 |
| Flash point | > 113°C |
| Autoignition temperature | 225 °C |
| Lethal dose or concentration | LD50 (oral, rat): 1250 mg/kg |
| LD50 (median dose) | LD50 (median dose) of N-Benzylimidazole: "1600 mg/kg (rat, oral) |
| PEL (Permissible) | Not established |
| REL (Recommended) | 50 to 100 mg/L |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Benzimidazole Imidazole N-Methylimidazole 1-Phenylimidazole 1-(2-Pyridyl)imidazole |