N-Aminopyrrolidine didn’t just pop up in a vacuum. In the mid-twentieth century, scientists started digging into the structure and behavior of small nitrogen-containing ring molecules. Chemists found pyrrolidine’s framework useful because it can be turned into a building block for more complex pharmaceuticals and industrial compounds. N-Aminopyrrolidine hit the scene as researchers tried different tweaks, swapping out parts of the ring to test new ideas. Labs worked with limited gear and couldn’t pull off the fancy analyses we have now, but they stuck with simple reactions, building off plain old pyrrolidine and figuring out how to stick an amino group on. Fast forward, and N-Aminopyrrolidine shows up in modern research, riding on decades of lab work and trial-and-error.
N-Aminopyrrolidine stands out mostly for its versatility in chemical synthesis. It offers a compact, amine-rich framework with both reactive nitrogen atoms sitting close together. That set-up makes it a handy tool for making drugs, specialty polymers, and agrochemicals. It slides right into various chemical reactions and adjusts to fit different downstream needs. In industry, people keep looking for chemicals like this, where a simple change in the structure gives access to a whole new class of compounds. That’s why chemists keep N-Aminopyrrolidine on their shelves, especially if they are digging for new molecules with pharmaceutical punch or targeting new classes of fine chemicals.
Pick up a vial and N-Aminopyrrolidine likely greets you as a colorless to pale yellow liquid or sometimes a crystalline solid. Its melting and boiling points fall right where small molecular amines usually land, low enough for routine handling but not so low that the stuff just evaporates off your lab bench. The molecule packs two nitrogen atoms in a five-membered ring, making it eager to either donate or accept protons. It dissolves in water and most organic solvents, letting you use it in a range of chemical setups. N-Aminopyrrolidine brings a whiff of that typical amine smell—a reminder never to ignore the fume hood.
On a chemical supplier’s shelf, the label reads something like “N-Aminopyrrolidine, ≥98% purity.” Sometimes you’ll see more specifics: CAS number, hazard warnings, and recommended storage temperature. The product label usually notes the chemical formula (C4H10N2), molecular weight, and batch number. Lab safety standards prefer this kind of clarity, especially with energetic amines, so you can see at a glance if you’re holding the right stuff and what kind of PPE to suit up with. Storage directions matter too—standard advice says keep the bottle in a cool, ventilated place and seal it tight to limit exposure to air and moisture.
In practice, N-Aminopyrrolidine grows from straightforward organic reactions. Start with pyrrolidine as your backbone. Reacting it with chloramine (NH2Cl) can pop the extra amine onto the ring. This approach needs a base present to trap the acid byproducts. Some researchers prefer to run amination directly using O-alkylhydroxylamines or other nitrene sources, which changes the yield and purity but offers a gentler run in some set-ups. Chemists always have an eye out for side products—things like diaminated pyrrolidines or ring-opened versions. Getting the highest purity means controlling temperature, rate of stirring, reagent addition, and clean-up steps. For industrial production, companies lean on continuous-flow reactors to handle larger volumes while keeping the product stream steady and limiting unwanted byproducts.
N-Aminopyrrolidine doesn’t sit still in a flask. The ring system wants to react, especially under mild heating or with the right catalysts. The molecule acts as a nucleophile, jumping into acylation and alkylation reactions. This opens up routes for making ureas, hydrazines, and diverse heterocycles. When you bring strong oxidants or acids into play, the amine group can be kicked off, replaced, or rearranged. Adding specific protecting groups shields the amine for later selective reactions, a tactic that shows up in pharmaceutical synthesis. The product’s predictability matters: once you’ve run a few modifications, you know what to expect from the next batch. Chemists use this to design pathways from simple building blocks to multi-stage synthesis of drug candidates or agrochemicals.
Chemicals rarely travel through research circles under a single name. N-Aminopyrrolidine goes by other tags: 1-Aminopyrrolidine, 2-Pyrrolidinamine, Pyrrolidin-1-amine, or just aminopyrrolidine. Suppliers sometimes stick to systematic nomenclature, but trade names or catalog codes make it into lab speak. This jungle of naming highlights the importance of clear labeling and cross-checking CAS numbers (for N-Aminopyrrolidine, it’s usually 447-85-6) to avoid mix-ups—nobody wants to waste a run with the wrong starting material.
Handling N-Aminopyrrolidine falls squarely in the realm of chemical safety you pick up in any working lab. Liquid or vapors prick at your eyes, nose, and throat. Avoid open bottles and always dial in the fume hood. Gloves, goggles, and lab coats stand between you and skin or respiratory irritation. If you toss N-Aminopyrrolidine into a reaction with oxidizers or acids, keep an eye on the heat since energetic amines can boil or catch fire. Store this stuff tightly sealed in cool spots, away from acids and moisture. Disposal goes through chemical waste channels—never down the drain—since local environmental regs take amines seriously. Working in pharma, I’ve seen enough slips to know that even mild-mannered compounds deserve full PPE and careful tracking from start to finish on the bench.
The real strength of N-Aminopyrrolidine lies in its application to synthetic chemistry. Drug companies reach for it to build rings in experimental pharmaceuticals—especially ones looking to tweak central nervous system action. Agricultural research looks at its potential for new pesticides. In material science, the amine groups open doors to custom polymers, sensors, and specialty coatings. Academic labs put it in the middle of mechanistic studies, checking how amine reactivity affects more complex molecule formation. Every year, a handful of papers push the boundaries, using N-Aminopyrrolidine to forge new molecular frameworks, map out synthetic shortcuts, or hunt for biological activity from novel molecules.
R&D teams demand reliability and adaptability in their reagents, and N-Aminopyrrolidine brings those qualities. I’ve worked in teams where access to such a flexible amine made the difference between running a stalled project and discovering a breakthrough pathway for a key intermediate. Finding new ways to adjust the amino group—adding a methyl or protecting it against unwanted reactions—fuels further research. Companies try to squeeze more out of one pot reactions, cut down on toxic byproducts, and redesign old syntheses for greener, cheaper, faster results. Universities partner with suppliers to fine-tune purity levels, stabilize shelf-life, and develop new analogs modeled on N-Aminopyrrolidine’s backbone. The chemical’s presence in patent filings shows its growing footprint, more than just another reagent—it’s a platform for creativity in synthetic labs.
Toxicity always looms in new chemical development. Researchers study N-Aminopyrrolidine in cell tests and animal models, tracking irritation, mutagenicity, and metabolic stability. Most recent open data show modest toxicity at usual lab concentrations but flag up respiratory and skin sensitivity with larger exposures. Glove and fume hood protocols make sense for a reason—amines get absorbed fast and can toss up allergic responses, especially after repeated use. Teams look for acute and chronic impacts, watching for signals that could block its use in pharmaceuticals or specialty chemicals. Regulatory limits on workplace exposure have driven more studies, aiming to understand long-term risks and avoid problems downstream if the compound sees wider adoption.
N-Aminopyrrolidine keeps drawing fresh attention year after year. As green chemistry look to replace old, bulky reagents, this unfussy scaffold offers a chance to streamline synthetic routes and make products safer for both workers and the environment. The rise of personalized medicine stokes demand for flexible building blocks—some of which could spring from this simple ring. Advances in catalysis and flow chemistry could unlock even cheaper, more sustainable production, folding N-Aminopyrrolidine deeper into daily use in pharma and industry. Investments in toxicology will smooth the way for new regulatory approvals, turning basic research into practical products. In research and real-world labs alike, chemists stay tuned for fresh applications and better ways to use this quietly powerful molecule.
Plenty of chemicals float around in labs, but some names rarely leave the textbooks until they start showing up in patents and research updates. N-Aminopyrrolidine does just that. Tucked in the toolbox of organic chemists, it plays a key role in piecing together new molecules. From where I sit, the usefulness lies in its knack for creating nitrogen-rich building blocks, reshaping how drug makers and material researchers build the next wave of pharmaceuticals.
Drug discovery stands out as an area hungry for small tweaks that lead to big changes. The pharmaceutical crowd likes molecules that can carry an amino group in just the right spot, as those can determine whether a drug binds to a target or never gets a chance. Having worked alongside a few colleagues focused on medicinal chemistry, I’ve watched them reach for clever reagents that do one thing really well—add or move a part of the molecule without setting off a chain of problems.
N-Aminopyrrolidine falls into this sweet spot. Its structure makes it a useful “nucleophile” in several key reactions. In plain terms, it’s willing to jump into a chemical reaction and share its electron pair, helping shape new rings or add side branches on bigger molecules. These tweaks can decide if a drug becomes more soluble, or if it slips through the cell’s defenses and gets to work. Some reports mention its hits in research programs targeting cancer and infectious diseases—both areas where having new scaffolds can mean the world.
Pharma isn’t the only frontier where folks put N-Aminopyrrolidine to work. Polymer scientists like having small, stable nitrogen-containing units that can anchor onto larger chains. In coating and plastics research, adding these sorts of groups changes how materials interact with water, light, or even bacteria. Whether you’re tuning insulation for electronics or making a coating that resists scratches, tailoring the chemistry at this simple building-block level pays dividends down the line.
Some labs use it for preparing ligands—small bits that grab onto metals—and these end up as critical players in catalysis or in medical imaging. With experience running synthetic routes in a small-scale setting, I’ve learned that having a toolkit with chemicals like this can shave weeks off the time spent testing ideas, letting people focus less on repeated setup and more on creative problem-solving.
Using N-Aminopyrrolidine doesn’t come without a headache or two. Handling amino chemicals brings safety, storage, and disposal challenges. It’s not uncommon to see researchers grappling with regulations around hazardous waste, and for smaller shops, that can eat up plenty of resources. Looking ahead, I think anyone involved needs to keep pushing for safer handling methods and greener alternatives. Replacing harsh reagents, finding milder conditions, and making process steps less toxic will serve everyone better.
The supply chain question pops up as well. The world found out in the past few years how tricky it gets finding specialty chemicals in a crisis. Domestic manufacturing and robust sourcing connections aren’t really negotiable, especially for pharmaceutical and advanced material work, so keeping those lines healthy turns out to be as critical as any chemical breakthrough.
N-Aminopyrrolidine stands out in modern chemistry labs, quietly enabling progress across disciplines. It’s not the flashiest of chemicals, but few on the shelf impact research pipelines quite as directly for both medicine and materials.
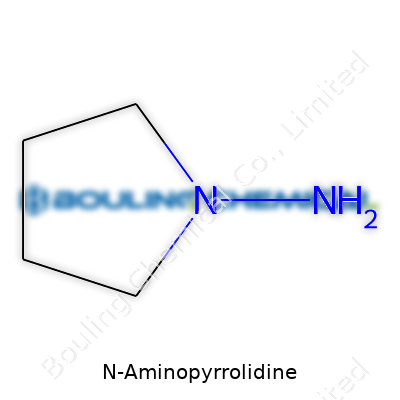
N-Aminopyrrolidine isn’t a big name outside chemistry labs, but this little molecule holds its own in the world of organic compounds. Its skeleton centers around a pyrrolidine ring—a five-membered ring made of four carbons and one nitrogen. Give it an amino group stuck to the nitrogen, and you’ve got N-aminopyrrolidine. It sounds simple, but these details open the door to a load of interesting chemistry.
Chemists write its formula as C4H10N2. Picture a five-sided ring—four corners belong to carbon atoms, one to a nitrogen atom. To lock in the “N-amino” part, a -NH2 group connects right onto that ring nitrogen, creating a structure with two nitrogens. Each carbon carries its full complement of hydrogens, so the ring stays saturated—there’s no double-bond business muddling things up.
People new to the scene might shrug at this, but the presence of two reactive nitrogen spots brings value, especially for folks who build bigger, more complex molecules. That N-amino group doesn’t just hang around looking pretty—it’s looking to react. Synthetically, this opens up all kinds of possibilities, allowing chemists to build out larger frameworks, make new heterocycles, or tweak drug molecules with extra specificity. I remember poking at amine chemistry in college and marveling just how small changes—like moving an NH2 group—led to wildly different chemical behaviors.
N-Aminopyrrolidine’s chemical structure means more than a name on a page. In the lab, its dual nitrogen setup finds real work in creating pharmaceutical intermediates and agrochemicals. Sometimes, it helps build up molecules that fight infections or pests. Its ring forms part of bigger frameworks in a range of designer drugs.
If you dive into patents, you’ll notice this motif popping up in places where researchers want to create new compounds without reinventing the building block wheel. Its role isn’t always as the headliner, but its structure plays supporting roles where reactivity and a small, nitrogen-rich core matter.
One issue stands out: controlling side reactions. That extra amine likes to react with loads of stuff, so managing purity and getting only the product you want can turn into a headache. Some labs chase ways to protect the nitrogen, only exposing it when needed. Synthetic routes can get creative, but putting that flexibility to practical use takes some planning and skill.
On a personal note, seeing the molecule’s layout reminds me how powerful small changes in structure can be. As interest in new medications and agricultural tools grows, it makes sense to keep exploring small heterocycles like N-aminopyrrolidine. Whether it’s a stepping-stone toward a pharmaceutical breakthrough or a handy rung on the chemical synthesis ladder, there’s no denying this compact molecule matters.
N-Aminopyrrolidine isn't a household name, but anyone who’s worked in a chemistry lab or industrial setting knows that chemicals like this one demand respect. It has a reputation for being a little unpredictable and potentially harmful. I've seen what happens when safety corners get cut: stains on benches that nobody can explain away and a whiff of chemical in the air that stings your nose for hours. It's a wakeup call for how you approach these tasks.
Think of gloves, goggles, and those long lab coats as your daily armor. The skin absorbs things faster than most folks imagine, especially chemicals like N-Aminopyrrolidine. I've seen coworkers shrug off gloves for just "a minute" and come away with red, irritated fingers. Splash-resistant goggles guard your eyes from sudden reactions—nobody wants to deal with an emergency eyewash station. Plenty of people underestimate the need for lab coats, but coveralls or even an old shirt won’t cut it. Cheap gear can save your skin today and headaches later.
No chemical smells friendly, but something about N-Aminopyrrolidine’s fumes makes you pay attention fast. Breathing that stuff in isn’t only uncomfortable—it risks long-term lung issues. Always handle it in a fume hood or work outdoors if you have that luxury. Good airflow kicks up safety a notch and keeps exposure low. Once, our fume hood failed during a busy morning. We noticed the irritation and rushed to fix it quickly; the alternative could have been a lot worse.
Over the years, I’ve seen what happens when these steps get sloppy. Chemicals live on shelves in glass bottles that sometimes look almost identical. Label every container sharply with not just a name, but also hazard details. You don’t want distractions turning a safe day into a dangerous situation. N-Aminopyrrolidine stays reactive if stored near acids or oxidizers; keep it separate, in a tightly sealed bottle, far from sunlight or heat. The right label and storage can prevent a minor spill from becoming a bigger mess.
No one expects a spill, yet nearly everyone in a lab has faced one. Having an absorbent spill kit nearby saves panic and wasted time. A good rule: clean up small amounts quickly and methodically, never with bare hands or a kitchen towel. For bigger messes, evacuate and call in people trained specifically because improvisation helps nobody. Don’t ever wash chemicals like this down the drain—check disposal instructions and bag up waste for hazardous collection. Environmental rules have teeth for a reason.
Walking newbies through the dos and don’ts shapes habits more than any manual. Encourage questions, do drills, and review emergency contacts often. I’ve watched seasoned staff point out gaps in routines because everyone has blind spots. Real safety shows up through good habits and genuine teamwork. Chemistry isn’t forgiving if you go it alone or cut corners, but a culture that puts smart practices ahead of shortcuts keeps everyone safer and the work rolling along.
N-Aminopyrrolidine doesn’t turn up in every lab, but for those who handle it, safe storage matters. This compound has shown value in pharmaceutical research. The catch? It brings risks like most amines—flammability, reactivity, and some health hazards. Keeping things safe goes beyond putting a bottle on a shelf. The way chemicals get stored often decides whether you go home with all your fingers or not.
N-Aminopyrrolidine reacts with air and moisture. Leaving a bottle open even for a short time can let water vapor and oxygen sneak in. This isn’t just a point for a safety lecture; water contamination ruins purity, and oxygen increases fire danger. Glass with tight-sealing lids cuts down on these problems. In my own time working with reactive compounds, I learned that simple glassware beats improvising with leaky containers.
A bottle sitting out on a warm bench in the open sets up trouble. N-Aminopyrrolidine catches fire if the temperature climbs or if it touches something like acid. Store it in a cool spot, far from any source of heat. Never keep it close to acids or strong oxidizers. Anyone who’s ever seen a fume hood fire knows how fast things turn ugly once incompatible bottles share a cabinet.
Some labs use dedicated chemical storage rooms with temperature controls. I remember walking into one storage room after a long weekend where the air conditioning failed. Bottles sat sweating. Any volatile compound turns into a bigger risk quickly. Chemical safety officers constantly nag about separate cabinets for flammables, and they’re right. Flammable cabinets give you fire-resistant peace of mind. That matters if an accident ever happens.
Years ago, I watched an experiment go wrong just because a vent wasn’t turned on. Storing something like N-Aminopyrrolidine in a well-ventilated area isn’t up for debate. Accidentally opening a bottle can release irritating vapors. Fume hoods or ventilated storage cabinets pull those vapors away from your lungs, keeping the awkward emergency room visits out of your day.
Poor labeling caused more headaches than I care to recall. Once, a researcher grabbed the wrong amine and ruined a whole week of work. Bare minimum, a label gives compound name, concentration, and hazard warnings. Even better if the date gets slapped on. It may seem boring, but nothing speeds up a crisis like not knowing what just spilled.
Lots of folks underestimate small spills or think a cracked lid will do “just for tonight.” That’s gambling. A cracked lid can leak vapors, which won’t stay a secret if the bottle tips over. Also, never store in plastic unless you are sure about chemical compatibility. Certain plastics turn brittle or dissolve after a while. Glass beats plastic for a reason—it doesn’t react the way cheap polypropylene sometimes does.
Good storage doesn’t come from a fancy cabinet alone. Habits shape safety. Double-check seals, keep incompatible compounds apart, and make it a routine to replace old or suspicious containers. Take ten minutes at the end of the week for a rundown in the storage area. That habit has saved more than one experiment from disaster—and probably kept plenty of labs from disaster drills turning real.
Every so often, a compound crops up that feels both familiar and overlooked. N-Aminopyrrolidine fits that bill. It comes as a clear or pale yellow liquid, not the type of stuff you’d spot on a store shelf, but more likely found tucked away in a research lab. With a chemical formula of C4H10N2, this molecule belongs to the family of aminopyrrolidines, sporting a five-membered ring where an amino group clings to the nitrogen atom. You might catch a faint whiff of ammonia if you get close, though hopefully in a properly ventilated space.
People who handle N-Aminopyrrolidine quickly learn its less-than-glamorous side. This compound, though a liquid at room temperature, doesn’t flash off at the drop of a hat. Its boiling point typically falls around 200°C. If you spill some, expect a mess—it’s quite soluble in water. The stuff mixes easily, which plays into how researchers put it to work.
You don’t want to forget the flammability. Even though it doesn’t catch fire as easily as diethyl ether, it’s still on the flammable side. That means no open flames, no carelessness. You suit up with gloves and goggles because N-Aminopyrrolidine can get under the skin, literally, causing irritation or worse if you don’t watch out.
N-Aminopyrrolidine brings a mix of basicity and reactivity. The lone electron pair on the nitrogen atoms means the molecule acts as a decent base, ready to grab protons in a snap. It also means this compound reacts with acids to form salts, which can be more stable for certain applications.
Another trait chemists value: the molecule’s willingness to take part in addition and substitution reactions. That amino group gets involved in making hydrazones or in building larger, more complicated molecules. For chemists figuring out new pharmaceuticals or pesticides, this kind of reactivity opens up creative routes to all sorts of structures.
The story behind N-Aminopyrrolidine matters because of its potential in drug design and synthesis. Researchers look for building blocks that can bring flexibility, and this compound fits the bill. It’s more than just a bench curiosity; it often pops up in the pathway when building up antiviral or neuroactive drug candidates. Given how labs always chase new antibiotics and cancer treatments, a molecule providing new handles for creativity holds value.
Still, industrial scale-ups demand caution. Its flammability, toxicity, and potential for side reactions call for careful handling. Facilities using this compound invest in closed systems and protective equipment to keep everyone safe. On a practical note, strengthening lab safety protocols, improving storage containers, and regular training go a long way to prevent mishaps.
For those invested in cleaner chemistry, the water solubility turns into both an advantage and a challenge. Easy mixing can help streamline reactions, but disposal turns tricky because release into wastewater can hurt the environment. Here, investing in better waste treatment or swap-in biodegradable alternatives should remain a priority.
N-Aminopyrrolidine won’t make headlines, but its presence in the chemical toolbox keeps research moving. Stepping back, progress depends on staying sharp with safety, seeking greener processes, and recognizing the value of every tool on the shelf—even the ones with unremarkable names.
| Names | |
| Preferred IUPAC name | 1-Aminopyrrolidin-1-ium |
| Other names |
1-Aminopyrrolidine N-Aminopyrrolidine |
| Pronunciation | /ɛn-əˌmɪnoʊ-pɪˈroʊlɪdiːn/ |
| Identifiers | |
| CAS Number | 1008-73-7 |
| Beilstein Reference | 361873 |
| ChEBI | CHEBI:134411 |
| ChEMBL | CHEMBL463901 |
| ChemSpider | 141357 |
| DrugBank | DB08366 |
| ECHA InfoCard | 23-211-756-808-200 |
| EC Number | 223-014-7 |
| Gmelin Reference | 92956 |
| KEGG | C19238 |
| MeSH | D017967 |
| PubChem CID | 11934700 |
| RTECS number | UW2275000 |
| UNII | 00D8F7WS2Z |
| UN number | 2810 |
| CompTox Dashboard (EPA) | DTXSID4078976 |
| Properties | |
| Chemical formula | C4H10N2 |
| Molar mass | 87.14 g/mol |
| Appearance | White solid |
| Odor | Odorless |
| Density | 1.13 g/mL at 25 °C (lit.) |
| Solubility in water | Soluble |
| log P | -0.47 |
| Vapor pressure | 0.425 mmHg at 25°C |
| Acidity (pKa) | 12.17 |
| Basicity (pKb) | 3.03 |
| Magnetic susceptibility (χ) | -52.16·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.498 |
| Dipole moment | 1.49 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 243.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -59.6 kJ mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -4279.8 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | N05CM22 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes severe skin burns and eye damage. Causes serious eye damage. |
| GHS labelling | GHS02, GHS05, GHS07 |
| Pictograms | GHS05, GHS07 |
| Signal word | Danger |
| Hazard statements | Harmful if swallowed. Causes severe skin burns and eye damage. May cause an allergic skin reaction. Harmful to aquatic life with long lasting effects. |
| Precautionary statements | Precautionary statements: P261, P280, P305+P351+P338, P337+P313, P405, P501 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | 85°C |
| Lethal dose or concentration | LD50 (oral, rat): 78 mg/kg |
| LD50 (median dose) | LD50: 1000 mg/kg (rat, oral) |
| PEL (Permissible) | Not established |
| REL (Recommended) | 100 mg |
| IDLH (Immediate danger) | IDLH: Not established |
| Related compounds | |
| Related compounds |
2-Pyrrolidone Pyrrolidine N-Methylpyrrolidine Pyrrolidine-2-carboxylic acid N-Acetylpyrrolidine |