Decades back, organic chemists chased after molecules that could re-shape the way industry and academia approached both synthesis and drug design. N-Amino Ethyl Pyrrolidine found its way into research notebooks in the mid-twentieth century, in an era when synthetic amines brought fresh hope for pharmaceuticals and polymers. It didn’t rise to the spotlight overnight—its benefits unfolded step by step as researchers started grasping how tiny structural tweaks in cyclic amines could turn entire fields on their heads. Labs across Europe and North America laid the groundwork, documenting the properties of this molecular ring and evaluating activities that went beyond classic amines. Over the years, this molecule built a reputation, supporting studies that probed neurological and catalytic mechanisms and slowly earning a permanent place on chemical shelves.
N-Amino Ethyl Pyrrolidine presents a compact structure, striking a balance between chemical simplicity and reactivity. Its ability to bridge between hydrophilic and lipophilic environments makes it appealing whether applied in fine chemistry or as an intermediate for advanced compounds. The molecule brings together a five-membered ring—part of the pyrrolidine family—with an aminoethyl “tail.” This combination creates an adaptable scaffold, opening pathways to both pharmaceuticals and specialty additives. Commercial suppliers tend to handle it in small- to moderate-scale production, targeting researchers and industries who value its reactivity over sheer volume.
This amine shows up as a clear or pale liquid, with a faint but sharp smell much like other small organics in its class. It's reasonably soluble in water and most common organic solvents, which points toward both convenience and risk—the molecule travels easily during both intended reactions and spills. The basic (alkaline) nature of its amine groups pushes up the pH in aqueous solutions. With a low boiling point compared to bulkier heterocycles, labs working with N-Amino Ethyl Pyrrolidine notice volatility during distillation or heating steps. Its structurally exposed nitrogen atoms lend themselves to protonation and functionalization—a feature that’s not lost on those in R&D.
Quality matters when ordering chemical reagents, and this holds true with N-Amino Ethyl Pyrrolidine. Common technical sheets spell out concentration (almost always greater than 98% purity for lab stock), density, refractive index, melting and boiling points, and safety precautions. The label provides not just the chemical formula (C6H14N2) but also supplier-specific batch coding, hazard pictograms, and guidance on storage—usually cool, well-ventilated areas, away from acids or oxidizers. Import/export regulations flag it for attention given potential precursor uses in both research and illicit settings.
Bench chemists often build N-Amino Ethyl Pyrrolidine by coupling ethylenediamine with a protected pyrrolidine derivative. A typical approach might bring together N-protected pyrrolidine and bromoethylamine, letting them react in polar solvents under reflux, stripping off protecting groups in the final stages. Small tweaks—choice of solvent, temperature, catalyst, and order of addition—can drive up yields and reduce by-products. Some older routes relied on reductive amination or direct alkylation, but these less refined methods sometimes generated unwanted isomers. Recent green chemistry efforts have replaced harsh reagents with milder, less hazardous alternatives—though the central strategy of installing the amino ethyl group remains constant.
Having secondary and primary nitrogen centers, N-Amino Ethyl Pyrrolidine stands ready for a host of transformations. Acylation can turn it into amides useful in peptide research. Alkylation opens the door to more sterically hindered amines, widening its utility in complex synthetic schemes. Its ring nitrogen can entertain oxidations, leading to N-oxides, or reductions that trim down bulk for specialty ligands. Some transition metal-catalyzed couplings link it onto aromatic groups for advanced pharmaceuticals. In medicinal chemistry, modifying the aminoethyl or the pyrrolidine ring tunes binding and selectivity for receptor targets in drug candidates.
Catalogs offer N-Amino Ethyl Pyrrolidine under a handful of alternate labels. IUPAC refers to it as 1-(2-Aminoethyl)pyrrolidine. Chemical traders sometimes use 2-(1-Pyrrolidinyl)ethanamine. The systematic naming paints the structural relationships, but sometimes folks just call it “AEP.” In pharmaceutical literature, these synonyms shuffle between publications—leading newcomers to double-check CAS numbers before placing orders.
Working with N-Amino Ethyl Pyrrolidine invites caution. Exposure pathways—skin, eyes, inhalation—present hazards, much like with many amines. Direct contact may irritate skin and mucous membranes. Fume hoods and eye protection aren’t just formalities; too many chemists learn this lesson after a single spill or splash. Measures also cover storage away from combustibles and oxidizers, with engineering controls for handling vapors. Regulatory agencies mandate clear labeling reflecting its toxicity, and facility protocols require spill kits and neutralizing agents. Organizations embedding this chemical into their production flow sometimes invest in closed systems to limit vapor escape, pushing for higher operational standards across the board.
Pharmaceutical research counts on N-Amino Ethyl Pyrrolidine as a valuable starting material and building block. It nestles into drug candidate syntheses for central nervous system agents and enzyme inhibitors, and its structure offers a platform for countless analogs. Outside pharma, the molecule has a toehold in chemical biology, serving as a linker or functional azide precursor. Industrial processes tap its ability to tweak surface or catalytic properties for specialty polymers and adhesives. Academic labs adopt it for fundamental research—probing reaction mechanisms, serving as a base in catalytic cycles, and modifying it for structure-activity studies.
Ongoing projects dive into the reactivity of N-Amino Ethyl Pyrrolidine and its analogs. Large pharma companies and universities study how modifications affect receptor binding and metabolic fate, hoping to unlock treatments that dodge nasty side effects. Others focus on green routes, testing fermentation and biocatalytic approaches to shave off steps, waste, and regulatory headaches. Instrument firms explore its use as a calibration tool or label in chromatography and mass spec, exploiting its dual amine groups for cross-linking or derivatization. The spread of open access science pushes more detailed datasets into the public sphere, letting smaller players join in the innovation.
Safety officers and regulatory scientists take the toxicity of N-Amino Ethyl Pyrrolidine seriously. Animal-model studies show acute toxicity at moderate to high concentrations, underscoring the risk in both handling and disposal. Chronic exposure impacts neurochemistry and can produce lasting tissue changes, a finding common for molecules in the amine family. Environmental labs flag its persistence and mobility in water systems—biodegradation proceeds, but too slowly to ignore emissions. Some progress has come with improved detection methods in air and water, using sensitive mass spectrometric assays to catch even trace levels in workplace and downstream effluents. Industry trends step toward closed-system handling and rigorous personal protection, but even routine research environments keep a sharp eye on accidental releases.
The next decade promises both opportunity and scrutiny for N-Amino Ethyl Pyrrolidine. Medicinal chemists watch for analogs that could unlock new classes of therapeutics. Material scientists look for polymer modifiers or catalysts that draw on pyrrolidine’s unique ring features, aiming to build more durable or responsive coatings. Meanwhile, regulators prepare tighter oversight, responding to broader calls for environmental responsibility and workplace safety. Suppliers react with higher-purity offerings, eco-friendly packaging, and digital product tracking. Researchers across sectors keep exploring, betting that small changes in a molecule’s shape drive big shifts in performance. This active, ongoing role secures N-Amino Ethyl Pyrrolidine a firm place in tomorrow’s labs and manufacturing processes.
People outside of labs might never hear about N-Amino Ethyl Pyrrolidine, but anyone who’s spent time studying industrial chemistry or working with pharmaceutical precursors will recognize the name. In practice, most usage revolves around specialty synthesis. That means building blocks for other chemicals. N-Amino Ethyl Pyrrolidine sits at the beginning of processes that lead to medicines, surfactants, and sometimes even crop protection products.
Researchers find value in this compound for its reactive nature. The molecule brings both an amine and a pyrrolidine ring to the table. That gives chemists several routes for modification. In drug development, that’s gold. You can shift the structure a bit, throw in a side chain, or connect it to other molecules, all in pursuit of a new pharmaceutical candidate.
Building a new treatment usually leans on fine chemicals. N-Amino Ethyl Pyrrolidine often becomes one piece in a much bigger puzzle. Some antihistamines, local anesthetics, and certain central nervous system agents end up tracing their lineage to compounds just like this. The way its ring structure interacts with biological systems grabs the attention of medicinal chemists.
For anyone who’s read up on generic drugs, the science always points back to starting materials. Years spent watching chemistry teams test new molecules taught me that something as small as the amine group on this compound can turn an entire project. It’s not glamorous, but the progress of medicine slows to a crawl without consistent supplies of specialty precursors.
Not everything about N-Amino Ethyl Pyrrolidine connects to drug discovery. Agrochemical research teams use it when they’re hunting for better pesticides or plant growth regulators. The chemical features make it a useful candidate for tweaks that help target weeds or insects. One of my college mentors worked on herbicide formulations and always brought up how finding the right pyrrolidine derivative could shave months off the testing cycle.
Certain surfactants and specialty polymers come from this molecule too. Engineers in detergent and water treatment fields use surfactants to manage everything from laundry stains to wastewater emulsions. Flexible chemistry like this gives researchers room to design new cleaning agents. With rising concerns about environmental impact, safer and more degradable molecules hold more weight, and that’s another reason to explore pyrrolidine chemistry.
Producing N-Amino Ethyl Pyrrolidine requires careful quality control and safe handling. Like a lot of amine compounds, it needs proper storage away from heat and moisture. Chemical exposure regulations and safety data sheets aren’t the stuff of headlines, but attention to these details keeps workers and communities out of harm’s way. During production scale-ups, making the process greener stays on chemists’ minds. Switching solvents, optimizing reaction conditions, and investing in containment tech all help.
From what I’ve seen, collaboration between industry, academia, and regulatory bodies matters most. The more chemists share their results and safety data, the quicker everyone can pinpoint risks, improve synthesis efficiency, and reduce waste. In the future, stronger networks for sharing information could speed up approval and open up even more applications for this kind of nitrogen-rich building block.
N-Amino Ethyl Pyrrolidine, known in some labs as NEAP, is not as famous as aspirin or caffeine, but in chemistry circles, the name pops up, especially when designing molecules that need a flexible scaffold. The chemical formula for N-Amino Ethyl Pyrrolidine is C6H14N2. It comes from a structure built on a pyrrolidine ring—a five-membered nitrogen-containing ring. Add an ethyl group with an extra amino attached to the nitrogen, and there's your molecule.
Looking at this formula, you might shrug and think, "Just another organic molecule." Growing up, I thought all chemistry was just white powder in bottles, but molecules like this drive changes in fields like pharmaceuticals, materials science, and even energy storage. NEAP brings a lot of flexibility because that combination of amine groups and a pyrrolidine ring can grab onto other atoms, link into longer chains, or balance solubility and reactivity. Medicinal chemists pay attention to molecules like this since the amine groups can anchor or bridge parts of a drug that target the body’s enzymes or receptors.
I remember working in a research setting where small tweaks made a big difference. The presence of a secondary amine in N-Amino Ethyl Pyrrolidine means it can react with acids to form salts, open opportunities for more stable drug forms, or stick together with chains to create new polymers. Its ring system brings some rigidity but doesn’t lose flexibility, which lets scientists build custom molecules with specific shapes.
Handling molecules like NEAP always needs attention. Amines can irritate skin and eyes, and the compound’s strong smell isn’t just a lab joke — it points to volatility, which in turn means proper ventilation matters. From experience, even small spills demand quick cleanup and gloves at all times. Training in chemical hygiene saves more than just time; it prevents trips to the nurse or, worse, the ER.
The science world gets more interesting and challenging every day. There’s still ground to cover in improving molecule handling. Digital inventory systems, stricter labeling, and quick-access safety sheets cut down the chance for hazardous mistakes, especially with compounds like NEAP remaining on the shelf. Simpler, reliable waste disposal plans also save headaches.
Open sharing of experiences and genuine mentorship in labs matter so much here. I learned a lot by following more experienced chemists instead of just reading manuals. They taught me where shortcuts end up causing problems. These lessons carry over to anyone handling new chemicals for research or manufacturing—a grounding reminder that elegant formulas need careful respect in the real world.
N-Amino Ethyl Pyrrolidine sounds like a mouthful. In the real world, it’s a chemical that crops up in research labs, especially in pharmaceutical development and chemical synthesis. I’ve seen firsthand how people underestimate it just because it’s not listed in the news as a headline hazard. It’s not some benign staple like table salt. Chemicals in this class deserve real respect to avoid serious mistakes. Some managers will treat it as routine, but those who work on the bench know there’s no room for guesswork.
You can’t tell just by looking if a clear liquid has danger written all over it. N-Amino Ethyl Pyrrolidine brings irritation risks to skin, eyes, and lungs. If you splash it, the burning sensation lasts ages and the smell lingers in the air. Inhaling even small amounts can mean headaches and dizziness that ruin your day. The safety data sheet spells out its hazards—caustic nature, risk of respiratory distress, and long-term effects are all concerns.
I remember my early days, watching a colleague ignore gloves for “just a quick pipette.” They ended up with red, cracked skin for weeks. After seeing those blisters, you check your sleeves twice. This chemical acts fast—one dropped vial led to evacuation, not a quick mop-up. Stories in chemical safety forums are littered with regret after someone skipped basic PPE, only to end up in the emergency room. Current regulations don’t go easy on mistakes, and rightfully so.
Precaution isn’t just about fear. It’s about going home in the same shape you arrived. I always use butyl or nitrile gloves because latex breaks down surprisingly quick with pyrrolidines. Lab coats with decent cuffs—no rolled sleeves—stop splashes from sneaking onto arms. Safety goggles aren’t optional; after seeing one chemical splash ricochet, you learn to keep your eyes out of harm’s way. A face shield adds security if the task risks sprays or pressure.
Keeping good ventilation makes a world of difference. I work behind fume hoods for anything that produces vapors or dust. Spills need more than paper towels. Neutralizing agents specific to amines take the edge off before sweeping up. Waste goes into clearly labeled containers; I’ve seen what happens when forgot-to-label turns into a guessing game with hazardous waste sorting. You check your emergency shower and eyewash stations regularly—not because they look nice, but because fast access counts in an emergency.
Rules alone won’t protect anyone. Labs with a culture of speaking up see fewer incidents. It helps to ask questions, especially when working with chemicals you’ve never handled before. Experienced researchers walk newer staff through real scenarios, not just theory—what happens if someone spills, where to run, how to clean. Training refreshers matter. I saw one lab leader run monthly drills for spills and exposures, and the result was fewer injuries, even with new staff rotation.
Growth comes from paying attention and sharing lessons. Posting clear, up-to-date safety protocols near work areas keeps best practices top of mind. Lab supervisors make a difference by modeling safe habits, staying present during risky procedures, and checking every container is sealed and labeled. Investing in quality gear—gloves, goggles, fume hoods—proves cheaper in the long run than hospital bills or investigations.
Every chemical brings a story, and N-Amino Ethyl Pyrrolidine deserves respect. People working with it can keep themselves safe by making protection and good habits part of daily life, every time, without shortcuts.
N-Amino ethyl pyrrolidine does not grab the public’s attention, but anyone who works in a lab or handles chemicals knows that overlooking small details in storage can spell disaster. This compound, like many amines and organic chemicals, arrives with some baggage: strong odors, skin irritation, risk if inhaled, and possible reactions with moisture or incompatible substances. Misplaced trust in a rusty cabinet can lead to bigger problems than just a regulatory fine.
Locking up chemicals does not just follow a checklist; it keeps people out of the emergency room. Storing N-amino ethyl pyrrolidine in a cool, dry spot—with good air movement—makes sense the way putting on your seatbelt does. Humidity creeps in where ventilation lacks, and I have seen bottles sweat and labels peel off in old basements. When that happens, not only do you forget what’s inside, but you risk leaking fumes or worse, contamination. You do not want this chemical near acids, oxidizers, or foodstuffs. A sturdy, closed cabinet, labeled, above ground moisture, not next to the radiator or in direct sunlight, tells anyone walking by: “This stuff gets respect.” I learned this after a poorly labeled shelf turned into a labeling mystery hunt—no one enjoyed that day.
Labels make or break good chemistry practice. Good labels save you when someone rotates shifts or a fire marshal swings by. Keeping the date received, concentration, and clear product name right on the bottle spares headaches and saves lives. I have seen chemicals stored in containers marked only by tape and sharpie—one cleaning crew almost trashed a bottle by mistake. Industry guidelines and OSHA both expect clear, non-negotiable labeling on every primary and working container.
Every time you grab the bottle or transfer some compound, gloves, glasses, and a lab coat stop little accidents from becoming big ones. Splashes come with the territory, sometimes without any warning. Just last spring, a small spill on a gloveless hand led to a rash and a lost shift for a student. Keeping goggles and gloves nearby is not just a rule—it directly saves people from pain and medical bills. I cannot overstate the difference simple habits make.
Ordering in bulk feels efficient until you realize half the container sits unused, packed behind newer stock. Every year, I’ve watched materials expire and leak or degrade, causing headaches when it comes time to dispose safely. Small batch storage, checked every quarter, keeps surprises at bay. Use a logbook, digital or paper, and prevent the “forgotten chemical” phenomenon. Waste totals—and disposal costs—plummet when everything stays accounted for.
Anything can happen, no matter how careful you are. Eyewash stations, safety showers, and clear exits save time and lives. Practicing what everyone should do—spill kits, evacuation—builds muscle memory. A plan locked in a drawer, unread, helps no one. In my own labs, even one yearly drill made a difference. No one likes emergencies, but running through what to do once beats panic every time.
It only takes one careless moment to set off a chain of trouble. Most mishaps do not happen from malice, just from thinking, “it’ll be fine this one time.” Careful storage, smart labeling, and respect for the people who will come into contact with chemicals like N-amino ethyl pyrrolidine make work safer for everyone, from experienced chemists to the newest technician. You may not get an award for putting a bottle on the right shelf, but you might just prevent the next accident.
N-Amino ethyl pyrrolidine shows up in labs where both reliability and chemical integrity rank high. Throughout my years working alongside chemists and in procurement roles, clarity around purity figures often made or broke project plans. Labs typically ask for this compound at purities of 97% or above. This means very little room for impurities, which helps ensure reactions go the way they should without unexpected by-products. For anyone working with sensitive syntheses or building out pharmaceutical lines, even a small drop in purity can cause full batches to get scrapped.
Looking through product specifications from major suppliers like Sigma-Aldrich, TCI, and Alfa Aesar, the labels often read “>98%,” sometimes reaching for “99%.” These numbers aren’t just for show; they reflect batch testing under strict quality controls. Each batch attaches a certificate of analysis, showing the results of gas chromatography or nuclear magnetic resonance tests. Those details help researchers confirm what’s actually in the bottle. If purity dips below the common standards, side reactions and longer purification steps drain budgets and slow timelines.
Packaging plays a bigger role in the chemical world than most realize. N-Amino ethyl pyrrolidine doesn’t handle exposure to air too well. Moisture, in particular, can mess up its stability. Most suppliers bottle this amine in sealed amber glass containers. The brown tint blocks light, cutting down on the risk of UV breakdown during storage or transit. Screw caps lined with Teflon or similar inert materials help prevent leaks and chemical reactions at the lid.
Small-scale buyers like research labs often pick up this compound in quantities from 5 grams up to 100 grams per bottle. Larger volumes sometimes come in metal drums with inner linings, aiming at manufacturers who run bigger reactions. Each time I’ve ordered, I checked for tamper-evident seals and clear hazard labeling. Handling and safety information stays close by, too. Safety data sheets (SDS) and hazard pictograms stick right to the outer packaging—nobody wants to guess which bottle in the fridge carries a health risk.
Proper packaging keeps costs lower by cutting down spoilage and waste. I remember once when packaging broke down in transit, leading to loss for both the supplier and our research group. Reliable sealing and clear labeling help us avoid those setbacks. If temperature swings threaten the product, good suppliers send shipments with ice packs or insulation, though most N-Amino ethyl pyrrolidine handles room temperatures for shorter trips.
Traceability has become more important in recent years. Detailed batch numbers and date stamps now help labs meet GMP (Good Manufacturing Practice) guidelines. Auditors and regulators look for that documentation to confirm materials match what’s being reported in trial data or production logs. Mistakes in packaging or mislabeled purity don’t just mean wasted chemicals—they can slow down regulatory filings and put new drug projects at risk.
Some hurdles still make the process bumpy. Smaller labs struggle with minimum order sizes forcing surplus storage or higher costs per gram. Suppliers could offer more flexible packaging, maybe pre-weighed sealed ampoules or eco-friendly refill formats. Transparent third-party quality audits and digital certificates would also help researchers make smarter purchasing choices without waiting days for paperwork.
In short, N-Amino ethyl pyrrolidine shows how careful attention to purity and packaging can save a lot of time, money, and back-and-forth in the lab. Every step from manufacturing to labeling shapes the confidence that researchers and safety officers put into their work. Getting these details right keeps innovation moving forward.
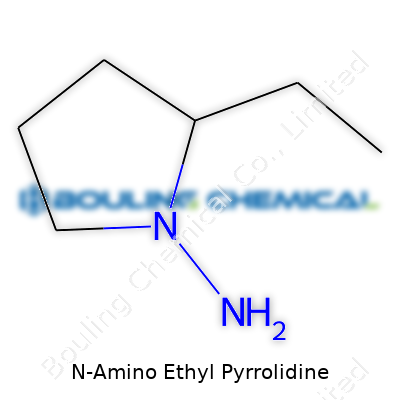
| Names | |
| Preferred IUPAC name | 2-(Pyrrolidin-1-yl)ethanamine |
| Other names |
1-(2-Aminoethyl)pyrrolidine 2-(Pyrrolidin-1-yl)ethanamine Aminoethylpyrrolidine 2-Pyrrolidinylethylamine |
| Pronunciation | /ɛn-əˈmiːnoʊ ˈɛθɪl pɪˈroʊlɪdiːn/ |
| Identifiers | |
| CAS Number | 120-94-5 |
| 3D model (JSmol) | `CCCC1N(CCN)CCC1` |
| Beilstein Reference | 120924 |
| ChEBI | CHEBI:187145 |
| ChEMBL | CHEMBL52559 |
| ChemSpider | 167361 |
| DrugBank | DB16245 |
| ECHA InfoCard | 03b7e2e2-29a3-4ee6-80f5-9bfc892e6284 |
| EC Number | 25652-71-7 |
| Gmelin Reference | 6036 |
| KEGG | C06017 |
| MeSH | D017211 |
| PubChem CID | 136352 |
| RTECS number | UF8050000 |
| UNII | F19L58106A |
| UN number | UN2810 |
| CompTox Dashboard (EPA) | DTXSID7038701 |
| Properties | |
| Chemical formula | C6H14N2 |
| Molar mass | 114.19 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | Amine-like |
| Density | 0.947 g/cm3 |
| Solubility in water | soluble |
| log P | -0.5 |
| Vapor pressure | 0.46 mmHg (25°C) |
| Acidity (pKa) | 10.75 |
| Basicity (pKb) | 4.41 |
| Magnetic susceptibility (χ) | -7.9 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.4840 |
| Viscosity | 2.48 mPa·s (at 20°C) |
| Dipole moment | 3.25 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 265.57 J/mol·K |
| Std enthalpy of formation (ΔfH⦵298) | N-Amino Ethyl Pyrrolidine: "-95.6 kJ/mol |
| Pharmacology | |
| ATC code | N06BX10 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | Harmful if swallowed. Causes severe skin burns and eye damage. |
| Precautionary statements | Precautionary statements: P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-3-0 |
| Flash point | 70 °C (closed cup) |
| Autoignition temperature | 270°C |
| Explosive limits | Explosive limits: 1.7% (LEL) - 10.7% (UEL) |
| Lethal dose or concentration | LD50 (oral, rat): 360 mg/kg |
| LD50 (median dose) | LD50 (median dose): 460 mg/kg (oral, rat) |
| NIOSH | NIOSH: UY7700000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | Store below +30°C. |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
N-Methylpyrrolidine Pyrrolidine 2-Ethylpyrrolidine N-Ethylpyrrolidine N-Aminoethylmorpholine N-Aminoethylpiperidine |