Decades ago, chemists searched for a way to put the acetyl group onto histidine and other sensitive amino acids in peptides and proteins. N-Acetylimidazole made its debut out of this effort. Synthesized for the first time in the 1950s, it quickly sidestepped bulkier, less selective agents. Ever since, labs exploring protein chemistry have kept a bottle of this stuff within easy reach. My first time handling it, I remember feeling both relieved and cautious—relieved at its efficiency, wary of how rapidly it reacts. Its story stands as a kind of lesson in how small molecules have quietly nudged forward our understanding, not only of biochemistry, but also of how research tools emerge through trial, error, and a lot of peer conversations.
You’ll usually find N-Acetylimidazole—also called acetylimidazole—sold as a fine, off-white powder, capped tightly to keep moisture out. Labs use it mostly as an acetylating agent targeting alcohols, phenols, and amines. It slides easily onto a shelf next to DMAP, acetic anhydride, and other similar reagents. Its acetyl group transfer can happen in water, organic solvents, and even in certain tissue environments. That kind of adaptability explains its popularity both at the workbench and in industrial settings. The labeling almost always points out its purity (often above 98%), its batch—because even tiny contaminants can send projects off course—and its storage instructions: cool, dry, away from sunlight.
Crystals of N-Acetylimidazole hold together well, packing a molecular weight of 110.12 g/mol. They melt around 90°C, which means a standard incubator or water bath easily liquefies it. I’ve left it too close to a warm plate before, only to come back to a sticky, unusable puddle. Solubility varies depending on the solvent; ethanol and dimethyl sulfoxide swallow it up fast, but water gives more resistance, except at higher pH. Its chemical backbone features an acetyl group tagged to the N1 atom of the imidazole ring. The acetyl moiety is ready to jump to nucleophilic partners—alcohols, amines, or phenols—quick as a flash, making it famous for speed and selectivity.
Suppliers stamp every bottle with a CAS number (2466-76-4), purity grades, and a warning about hydrolytic instability. The powder loses punch in damp air and under strong light, so storage inside airtight containers stays non-negotiable. Specifications often stretch to list elemental analysis, moisture content, and recommended shelf-life. Labels cover the GHS pictograms for skin and respiratory irritation, making gloves and fume hoods an obvious necessity. SDS (safety data sheets) spell out what to do if it spills, catches fire, or finds its way onto skin. Details aren’t there just for legal reasons—they keep both seasoned chemists and new students from making rookie mistakes.
The process isn’t overly complex: Mix acetic anhydride with imidazole, usually under gentle cooling, and watch as the acetyl group migrates. Once you cool, dry, and grind the crude product, normal filtration and recrystallization pull out a pure, white solid. Labs watching costs might recycle leftover imidazole or distill used solvents to lower waste. Batch size matters, because the reaction throws off heat and an extra mole of acetic acid. Some facilities tweak stoichiometry to shift yield or purify the product without chromatography, making the process more sustainable for larger-scale runs. Years of trial and error in my own lab showed that slow addition of acetic anhydride keeps the reaction under control and avoids side products.
N-Acetylimidazole gets along well with a wide class of functional groups. It shines most when transferring acetyl groups to tyrosine, serine, or threonine residues in peptides. In organic synthesis, it smoothly acetylates alcohols without overdoing it and without creating colored byproducts. It also helps in protecting groups, because the acetyl can be removed under mild alkaline conditions, restoring the original molecule with good yield. It opens up possibilities for site-selective modification—a big deal if you need to poke and prod one particular piece of a protein. Some creative chemists have used it to tag phenols for studies in lignin chemistry, or to build libraries of chemical probes. Once, after running a reaction overnight, I saw how just a dusting of sodium bicarbonate let me extract pure acetylated product without nasty tar, cementing its reputation for straightforward workups.
You might see N-Acetylimidazole labeled as 1-Acetylimidazole, or sometimes just acetylimidazole. Trade catalogs toss in numbers like AI-15 or batch-specific labels, but chemists across the globe all mean the same compound. Occasionally patents refer to it using systematic nomenclature—N-(1H-imidazol-1-yl)ethanone—but most stick with two or three main names to keep things simple.
Acetylimidazole irritates eyes, skin, and airways, so the entire prep and use happens inside a fume hood. Goggles and gloves stay non-negotiable, even if all you’re doing is weighing a few milligrams. Any spills get mopped up with dry paper towels, which then go straight into a sealed container marked for organic waste. In my own early experiments, a careless opening of the bottle resulted in stinging eyes and a runny nose—not an experience new researchers forget. Fire risk stays moderate; it’s no peroxide, but a strong oxidizer nearby raises real concerns. Disposal means collecting solutions into labeled waste containers, since dumping into the sink puts both people and the environment at risk—strict local rules make sure of that. Trained users rely on regular audits, safety briefings, and emergency drills to keep standards at the top of the checklist.
You’ll find N-Acetylimidazole at work in biochemical labs, especially when scientists need to pinpoint active sites in enzymes. Medical research groups rely on it for selective tagging experiments, often pushing boundaries on diagnostics and drug delivery. Pharmacies use it as an intermediate in some custom syntheses for rapid drug prototyping. Lignin researchers depend on it to mark phenolic groups, tracing decomposition pathways. In recent plant biology studies, the reagent helps map where phenol groups live in complex cell wall networks. Its ability to react quickly under gentle conditions fits high-throughput screening just as much as slow, careful structural studies. I’ve watched colleagues use it to tailor-make probes and test protein conformations, showing how versatile one simple compound can be.
Current research pushes for more targeted acetylation by tweaking pH, adding co-solvents, or playing with catalyst concentrations. By exploring these boundaries, scientists find links between acetylimidazole reactions and disease pathways, giving rise to new screening tools in drug discovery programs. Some groups chase greener synthesis by developing recyclable catalyst systems to cut down waste and improve selectivity. The drive to acetylate complex, sensitive molecules with fewer side reactions keeps this compound center stage in chemical innovation. On my own bench, even tiny changes to procedure produced big shifts in product isolation—proof that the journey from “good enough” to world-class often lives in the small details.
Testing on animals and cell cultures shows that N-Acetylimidazole poses risks at high concentrations, causing irreversible cell damage and tissue injury if handled without proper gear. Long-term exposure can attack the respiratory system, leading to symptoms from moderate irritation all the way to more serious outcomes. Most animal studies use short-term dosing and report acute toxicity without chronic buildup, but gaps remain—especially at the intersection of dose and duration. My own work with cell lines required extra controls to double-check for off-target effects, since even minor contamination throws off viability results. Science keeps moving forward, and so do the safety standards, with newer protocols putting in stricter exposure limits and better monitoring of workplace air quality.
Interest keeps growing in finding variants of N-Acetylimidazole that hit players other than imidazole rings—aromatic amines, heterocycles, and more. Green chemistry advocates push for reactions with renewable acyl donors, or swaps that recycle the imidazole moiety. Biomedical application leads researchers to design analogs that cross biological membranes with more control, improving both specificity and safety. As data science starts to guide acetylation site selection, chemists mix robotics and machine learning to predict which proteins or polymers benefit most from selective tagging. With regulatory agencies tightening standards, any advance that reduces toxicity, waste, or process steps makes a big impression. From history lab stories to current patent races, N-Acetylimidazole continues to power discovery and create new questions for tomorrow’s researchers.
In labs and factories, N-Acetylimidazole isn’t some household name, but it sure does get targeted attention from researchers and those working in the pharmaceutical industry. The main reason? This compound doesn’t just sit quietly in a bottle. Scientists look to it to help make key changes to other molecules, especially proteins. You see, a lot of experiments need a way to modify proteins at specific points, and this is where N-Acetylimidazole steps in.
N-Acetylimidazole serves as an acetylating agent. The business of "acetylation" means adding an acetyl group to another molecule. You grab a protein, treat it with this reagent, and it sticks an acetyl group onto a specific site—often a tyrosine or lysine residue in the protein chain. Suddenly, you’ve changed how that protein behaves. Biochemists rely on this tactic to understand all kinds of things: enzyme activity, protein folding, protein interactions. This helps not just in deciphering how life works but also in designing new drugs and treatments.
Pharmaceutical companies don’t skip over tools like N-Acetylimidazole. Sometimes a medicine’s development hinges on small changes to a molecule. Even vaccines or diagnostic tools sometimes call for proteins with altered functional groups. My time working with researchers in protein engineering taught me just how critical it can be to have reliable and quick acetylation methods. One time, a research project on enzyme inhibitors got bogged down until someone suggested N-Acetylimidazole – the whole pace of the work changed overnight. Fast, predictable modification gave clear results, letting ideas move from the bench to the paper, and sometimes toward actual patient treatments.
Not all the action happens in pharma or diagnostics. The food industry monitors proteins too, especially for allergens or for studying spoilage. N-Acetylimidazole makes it possible to track protein changes or block certain reactions. It helps keep people safe from hidden risks in processed foods.
Making acetylated proteins also spills into agricultural science. Improving the nutritional value or shelf stability of crops often includes fiddling with proteins. Techniques developed with N-Acetylimidazole in the lab sometimes move out into practical crop science, showing how connected chemistry is to daily living.
Working with chemicals like N-Acetylimidazole can turn messy. It reacts with water, so you often need dry, carefully controlled lab conditions. That raises cost and lowers convenience. If you breathe it in or it gets on skin, it can cause harm, so safety gear and training become mandatory. I’ve seen projects slowed down because of ventilation issues or delays just caused by not having the right safety data sheets ready.
Some researchers look for alternatives that cause less trouble. There’s always a push for greener and safer reagents. If labs move away from volatile or dangerous chemicals, the hope is that new discoveries won’t put health or the planet at risk.
Turns out, paying attention to workplace safety isn’t just about obeying rules; it makes research possible. Investing in the right safety gear, planning experiments carefully, and keeping protocols simple and clear makes using challenging chemicals manageable. Supporting the search for more sustainable or less reactive acetylating agents looks smart long term. Open sharing of safer alternatives, both in published papers and in day-to-day lab notes, helps people learn faster and stay safer.
N-Acetylimidazole isn’t likely to vanish from labs anytime soon, but the conversation is shifting. People value its usefulness, but nobody wants progress at the cost of safety or the environment. That balance keeps science moving forward, one experiment at a time.
N-Acetylimidazole sounds like something you would hear in a chemist’s jam session, and maybe in a way, it is: a small molecule with the ability to transform other compounds. Its chemical formula—C5H6N2O—describes a simple arrangement, yet this molecule manages to pull off impressive chemical tricks. At its core, N-Acetylimidazole brings together an imidazole ring and an acetyl group. That combination is more than just chemistry on paper; it turns into biochemical action.
Picture the imidazole ring as a five-sided structure, sort of like a pentagon in miniature, holding two nitrogen atoms. Those nitrogens have a particular knack for making bonds. Stick an acetyl group—think two carbon atoms with an oxygen double-bonded to one—onto the nitrogen at position one of the ring, and you end up with N-Acetylimidazole. Chemists draw it with the acetyl swinging off that top nitrogen, marking its favorite resting spot.
I remember my first hands-on lab with this molecule during undergrad. The instructor used it to show us how a simple change in structure could mean the difference between something that sits on a shelf and a compound that moves reactions along. N-Acetylimidazole works as an acetylating agent, meaning it hands off that acetyl group to other molecules, bumping up their chemical activity. Researchers lean on it to add acetyl groups to amino acids and proteins, especially when regular methods turn out too aggressive or messy. Its moderate reactivity lets you keep the fine details in complex biological experiments.
Ask anyone in the field, and you’ll probably hear how this substance plays a quiet yet vital role in biotechnology and pharmaceutical work. Drug researchers use it for acetylation of proteins and peptides—think swapping a hat from one Lego minifig to another, except that "hat" can control how a whole protein functions in your cells. In organic synthesis, its controlled acetylation helps create well-defined products for advanced medicines.
Talk to folks working with the stuff, and the conversation often circles back to one thing: safety. N-Acetylimidazole reacts not just with the targets you want but also with water and even skin. Accidental exposure brings real risks, and I’ve heard stories from people who learned the hard way about proper lab protocols. Fume hoods, gloves, and knowledge of what you’re handling go a long way here. Waste disposal also deserves attention. Since small molecules like this can turn up trouble in waterways, proper chemical waste collection isn’t just a bureaucratic box to tick—it’s something we owe to the people downstream.
Scientists keep digging for methods to make N-Acetylimidazole safer and easier to use. Some labs experiment with newer solvents or greener chemistry routes, aiming for reactions that get the job done but leave less mess and risk. Automation also helps limit direct handling, letting machines shoulder some exposure. In a time when lab safety and efficiency matter as much as discovery itself, a smart approach to molecules like N-Acetylimidazole keeps labs running and discoveries rolling in.
N-Acetylimidazole doesn’t show up in conversation much outside of chemistry labs. For most people, it exists only as a label on a container or a line in a protocol. Yet, in places where it’s used, the risks it brings stay very real. I’ve spent enough afternoons smelling sharp chemicals or reading hazard sheets to know that walking past storage guidelines can leave someone with more than a bad day.
This chemical lifts its head as an acylating agent, which means it reacts fast with even mild moisture or traces of water and alcohols. If the container isn’t sealed tightly or someone leaves it open on the workbench, you might end up with a product you can’t trust. Sometimes, forgetting airtight storage means an experiment flops or, worse, your safety takes a hit. Breathing in the dust or vapors often brings headaches, coughing, skin burns or eye irritation. Most folks don’t know that small mistakes build up and sometimes send entire teams scrambling to decontaminate the lab.
I’ve stood in enough labs to learn that sloppiness in storage gives chemicals a chance to ruin tools and threaten health. N-Acetylimidazole asks for dry, cool, and dark conditions, away from strong acids, bases, or oxidizers. Sticking it beside incompatible stuff—like bleach or acids—collects trouble. Humidity and light chip away at the chemical’s stability. Putting it on a high traffic shelf or near a heat source looks like a shortcut but invites problems later. Something as simple as a missing label or a cracked cap leaves everybody guessing about safety.
In my own lab work, I found that a serious respect for personal gear means fewer skin rashes and fewer accidental trips to the eyewash station. Smile at safety glasses, laugh at lab coats or ignore nitrile gloves, and trouble arrives eventually. I also noticed that a well-ventilated fume hood isn’t just for show. Working with N-Acetylimidazole outside ventilation leaves residue hanging in the air where it doesn’t belong.
Some fixes don’t cost much and save everyone time. Double-check the seals on chemical bottles. Use desiccants in storage cupboards to fight moisture. Make sure labels stand out with the hazard class clear. Collect waste separately in marked containers. I’ve seen labs skip some of these steps because they seem fussy, but every near-miss or close call tells the same story—take the small steps every time, not just on inspection day.
Teach new team members why the rules exist instead of just handing out safety sheets. Stories stick longer than rules. Share the mishaps and what nearly went wrong. Rotate stock often so everyone gets a look at the storage habits and learns what right looks like. This creates a culture where the next person down the line can trust the workspace, not dread it.
Care around N-Acetylimidazole saves more than money or product—it keeps afternoons routine instead of frantic. Good storage, right gear, and habits grown through teaching and example close the door on avoidable accidents. That means more focus on meaningful work and less anxiety about what’s hiding in those brown glass bottles at the back of the cabinet.
N-Acetylimidazole shows up in more laboratories than most folks realize. You find it in the hands of people who take proteins or enzymes and want to figure out how those molecules behave in real environments. The chemical acts kind of like a tagging marker: it pokes at proteins, attaches a small acetyl group, and signals that a certain part of the protein was accessible to the outside world. That one trick helps biochemists work out where the business parts of proteins live.
In my time shadowing a biochemistry lab, acetylation came up each time we wanted to see which amino acids in an enzyme’s guts actually do the heavy lifting. With N-acetylimidazole, the process happens pretty gently. Researchers trust it to work without shredding the whole protein. This precision lets us draw clear conclusions about how medicines interact with their targets, which feeds directly into drug design work.
Outside academic circles, companies use N-acetylimidazole in pharmaceuticals and materials science. It doesn’t just label proteins; it transforms them. Give it to a batch of cellulose, and you get cellulose acetate out the other side — a building block for everything from photographic film to eyeglass frames. It acts quickly, which makes reactions easier to control compared to older chemical treatments.
Drug makers appreciate how selective this chemical is. Stick it in a process meant to modify a molecule at just one spot, and it rarely messes with the rest. That sort of single-minded attitude helps during the final steps of synthesizing a drug. There’s less cleanup, and fewer arguments over whether the product is pure. Less waste means more money saved, plus a lower impact on the environment.
Protein chemists spend a lot of time worrying over which amino acids are vital for a protein’s job. N-acetylimidazole makes it easier to pick out those parts one by one. Each modified amino acid stands out in analysis, showing how a protein’s structure supports its role. This tool becomes especially important in the crowded world of enzyme engineering, where even a single misplaced group can ruin months of work.
I’ve watched projects stall for weeks because an active site got blocked during a careless step of modification. Using N-acetylimidazole, that risk drops. The process feels a bit like using a highlighter with a fine tip, hitting just the key words and leaving the rest untouched. That’s worth a lot in a field where every mistake costs both money and reputation.
No tool is perfect. Acetylation by N-acetylimidazole asks for careful attention to reaction time and temperature, or else selectivity drops and side products pop up. Labs sometimes have to tweak solvents or add buffers to keep the process running smoothly. I’ve seen chemists debate whether to switch to more stable but less adventurous modifiers just to keep everything predictable.
There’s also the matter of scale: small-scale reactions work beautifully in glassware, but ramping up calls for fresh problem solving. Companies must balance speed, cost, and safety in larger batches. The solution often comes down to better mixing technology and smarter process control rather than switching chemicals outright.
Research keeps rolling. People look for ways to use N-acetylimidazole that cut back on waste and side reactions. The next step might be greener solvents or more precise delivery methods. As science asks for more and more complex molecules — especially in medicine and materials — this small acetylating agent will keep pulling more weight, always just a step away from the spotlight.
N-Acetylimidazole sits on many chemists’ shelves. It often pops up in synthesis and research, especially in biochemistry labs. This powdery substance helps attach acetyl groups to other molecules and features in the shaping of certain pharmaceuticals or protein studies. It’s not a household name, but for people who handle chemicals, understanding its risks goes way beyond glancing over a data sheet. Missing the small details can lead to real harm.
Exposure to N-Acetylimidazole brings up more than just lab headaches. Skin and eyes really feel the sting—direct contact leaves burns and irritation, even after quick spills. Accidental splashes in the lab, a misplaced glove or wipe, and someone instantly deals with bright red welts. It doesn’t smell strong, so no warning signal hits your nose as with some more pungent chemicals.
On top of that, it doesn’t take much to breathe in dust if a vial breaks or the powder billows while you’re pouring. Inhaling particles, even just a bit, can cause a scratchy throat, sneezing fits, and a cough that refuses to quit. After repeated exposure or careless cleanup, the lungs and airways don’t bounce back so quickly. Data shows N-Acetylimidazole is acutely toxic if swallowed, so chewing a sandwich with powder-covered gloves nearly guarantees a trip to the ER.
As a reactive chemical, it doesn’t just sit in a bottle waiting patiently. It can react with water, acids, or even some everyday lab solvents. This means an accidental spill in the sink, or washing a beaker without proper disposal, can send up surprising fumes or create by-products you do not want near your hands or face. I’ve seen reactions fizz and smoke while cleaning up after an experiment; you learn not to trust seemingly “quiet” substances.
Besides immediate health issues, there’s something long-term. Some studies link this compound’s by-products to possible genetic mutations. That’s reason enough to motivate most folks in the lab to keep exposures low.
Chemicals like this demand a solid respect for personal protection. In my own experience, gloves and goggles go on before the bottle comes out. It’s never smart to trust luck. Lab coats stay buttoned, and I always pick work stations near a fume hood, even if the room looks empty. That hood keeps invisible dust and vapors out of your lungs.
Spills get scooped with absorbent pads, not dry towels that throw powder into the air. Waste disposal goes in sealed bags marked for hazardous chemicals. Nobody wants to rummage through a regular trash can and get a surprise dust cloud.
Washing hands with soap and warm water doesn’t get skipped, even after glove use. Shared workspaces need clear labels and storage, away from acids and moisture. That avoids accidental mixing and dangerous reactions. If an accident happens, immediate first aid matters—rinsing eyes or skin with water for several minutes can minimize long-term effects.
Chemical safety isn’t just up to the company or college. Every lab worker, from undergraduates to experienced researchers, builds the routines that keep people safe. Training sessions that show real-life accidents—photos, stories, or even mock drills—stick with people much longer than slides full of warnings.
Switching to safer alternatives, when possible, reduces risk altogether. No chemical comes without hazards, but a culture of respect and clear rules means everyone finishes the day unharmed. N-Acetylimidazole, like plenty of lab tools, acts as a reminder that chemistry returns exactly the respect you offer it.
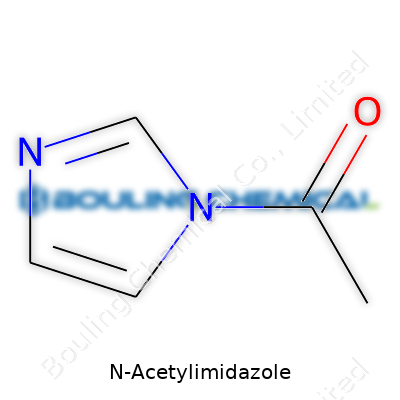
| Names | |
| Preferred IUPAC name | N-(1H-imidazol-1-yl)acetamide |
| Other names |
Acetyloxyimidazole N-Acetyl-1H-imidazole |
| Pronunciation | /ˌɛn əˌsiːtɪl ɪˈmɪdəˌzoʊl/ |
| Identifiers | |
| CAS Number | [2466-76-4] |
| Beilstein Reference | 353953 |
| ChEBI | CHEBI:74215 |
| ChEMBL | CHEMBL1231349 |
| ChemSpider | 15206 |
| DrugBank | DB08234 |
| ECHA InfoCard | 100.033.807 |
| EC Number | 211-500-2 |
| Gmelin Reference | 80856 |
| KEGG | C02577 |
| MeSH | D000580 |
| PubChem CID | 6996 |
| RTECS number | LW4250000 |
| UNII | U0SB639484 |
| UN number | 2811 |
| CompTox Dashboard (EPA) | DTXSID8022202 |
| Properties | |
| Chemical formula | C6H6N2O |
| Molar mass | 137.14 g/mol |
| Appearance | White to off-white powder |
| Odor | Odorless |
| Density | 1.204 g/cm³ |
| Solubility in water | slightly soluble |
| log P | -0.02 |
| Vapor pressure | 0.00981 mmHg at 25 °C |
| Acidity (pKa) | pKa = 12.5 |
| Basicity (pKb) | 7.0 |
| Magnetic susceptibility (χ) | -60.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.573 |
| Viscosity | 1.864 mPa·s (20°C) |
| Dipole moment | 3.73 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 354.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -221.8 kJ/mol |
| Pharmacology | |
| ATC code | N02BA21 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2-2-2 |
| Flash point | 101°C |
| Autoignition temperature | 245 °C |
| Lethal dose or concentration | LD50 (oral, rat): 940 mg/kg |
| LD50 (median dose) | LD50 (median dose): 990 mg/kg (rat, oral) |
| NIOSH | SN1975000 |
| PEL (Permissible) | PEL for N-Acetylimidazole: Not established |
| REL (Recommended) | F27 |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Imidazole Acetyl chloride N-Methylimidazole Benzimidazole 1,2-Dimethylimidazole |