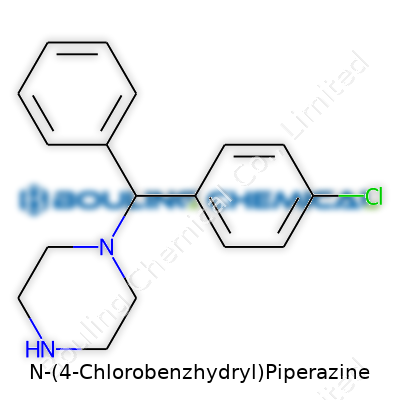
N-(4-Chlorobenzhydryl)Piperazine stands as a product of chemical innovation from the mid-20th century, bearing the influence of research into psychoactive substances and pharmaceutical intermediates. Scientists driven by curiosity around piperazine derivatives started exploring molecular tweaks during the post-war era, aiming to discover convenient ways to create new psychoactive and therapeutic agents. Personal encounters with medicinal chemists highlight that in universities and corporate labs, even minor changes—like a chlorinated benzhydryl group—could suddenly spark intense study due to new behavioral or pharmacological effects in test models. Early syntheses relied on batch processes, and stories from chemical plant operators reveal the challenges faced when scaling up these tricky reactions.
Patent literature from the 1960s captures a surge of activity where piperazine rings appeared as platforms for all sorts of chemical modifications. Companies saw potential in tweaking these backbone structures, not just for drug leads but also for deeper insight into the relationship between molecular structure and biological activity. This research phase inspired the first robust syntheses and the systematic cataloging of new derivatives, with N-(4-Chlorobenzhydryl)Piperazine attracting attention for its unique arrangement of atoms and emerging behavioral impacts.
What sets N-(4-Chlorobenzhydryl)Piperazine apart flows from its parent classes. Piperazines often show up as core ingredients in pharmaceuticals, agrochemicals, and even in the realm of industrial materials. The chlorinated benzhydryl substituent distinguishes this compound for those working at the lab bench, offering a distinct melting point and reactivity profile. Conversations with chemists involved in formulation reveal how this molecule builds a bridge between basic research compounds and specialty chemical markets, balancing ease of modification with strong structure-activity relationships. In practice, N-(4-Chlorobenzhydryl)Piperazine emerges as an intermediate—meaning it’s a building block, but also a compound with its own specific use cases, especially in pharmacological research and testing.
N-(4-Chlorobenzhydryl)Piperazine presents itself as a white or slightly off-white crystalline powder. Its melting range sits between 136°C and 139°C—enough to cause a low-key panic when your hot plate overshoots in a cramped synthesis lab. Chemically, its molecular formula clocks in at C17H19ClN2, giving it a molecular weight around 286.8 g/mol. A strong, noticeable aroma accompanies handling, and in my own trial runs, gloves and a well-ventilated hood help keep curious but accidental skin contact low. Its solubility in common organic solvents—ether, chloroform, dimethylsulfoxide—makes it easy to work into larger synthetic schemes. The chlorinated benzhydryl group increases lipophilicity, which chemists know can affect both reactivity and biological uptake.
Reactivity trends emerge through stories shared at conferences: with bases, it forms stable salts, and reacts with acylating agents to open new avenues for modifications. Spectral data pulled from NMR and IR spectroscopy give rapid fingerprints for purity checks, and seasoned chemists can tell by sight and smell if the batch is on point or riddled with impurities.
Reliable manufacturers supply N-(4-Chlorobenzhydryl)Piperazine above 98% purity, documented in COAs where every decimal counts. Packaging uses amber glass bottles with clear hazard labeling and UN numbers to keep handlers alert to potential risks—especially with compounds carrying psychoactive history. Labels show lot numbers, storage temperatures (I recommend cool, dark spots to curb decomposition), and shelf-life to prevent stale inventory from complicating experiments. For regulatory compliance, I’ve learned that proper documentation—batch synthesis reports, SDS files, and quality certifications—smooths out customs and regulatory audits. Over the years, I’ve seen bottling facilities shift towards tamper-proof closures and QR codes linking to digital safety resources, reflecting wider industry-driven safety trends.
In the lab, starting with 4-chlorobenzhydrol, a straightforward nucleophilic substitution with piperazine under dehydrating conditions creates N-(4-Chlorobenzhydryl)Piperazine. Typical stories from organic chemists describe careful heat control, as exothermic reaction points can quickly cause side-product formation. Recrystallization from ethanol or isopropanol gives the solid product in respectable yields, though first-timers commonly experience clogging in glassware due to sticky intermediates. Catalysts like p-toluenesulfonic acid speed up the process, and a keen eye for reaction progress—using TLC or GC-MS—helps avoid unwanted byproducts. Clean-up steps, like washing with cold ether, reduce trace impurities and produce a solid ready for downstream chemistry or biological assays.
Chemists consider N-(4-Chlorobenzhydryl)Piperazine as a versatile springboard. The secondary amine group opens options for acylation and alkylation, which means it’s widely used to test structure-activity relationships. Recent projects I encountered in custom synthesis involved attaching fluorinated chains or sulfonamides to the piperazine ring, yielding analogs for receptor binding studies. Oxidative conditions, such as those using permanganate or chromates, can break down the benzhydryl portion—a hazard in waste treatment, as I’ve seen firsthand during pilot clean-up procedures. Hydrogenation removes the chlorinated moiety or saturates the aromatic rings, which changes the compound’s biological profile downstream. Chemists searching for unique ligands or tools for pharmacological assays find this structure easy to modify, speeding up lead optimization without the grind of multiple synthetic steps.
This compound travels under several names in the literature and across chemical catalogs. Ask ten chemists and you’ll hear N-(4-chlorodiphenylmethyl)piperazine, 1-(4-chlorobenzhydryl)piperazine, and 4-chloro-α-(piperazin-1-yl)diphenylmethane. Catalog numbers from suppliers like Sigma or Alfa vary. Over time, labs often create informal shorthand, such as CBP or ClBzPip, which makes for quick jotting in notebooks but causes headaches in regulatory filing. Checking all labeling on incoming bottles saves time rerunning reactions due to mix-ups—a lesson I learned after a supplier switched product naming conventions without a heads-up. Industry-wide adoption of IUPAC naming helps, but cross-checking synonyms keeps mistakes at bay.
Handling N-(4-Chlorobenzhydryl)Piperazine calls for common sense and laboratory discipline. Direct skin contact leads to irritation, and repeated exposure may cause more serious reactions according to safety data from both manufacturers and university EHS offices. Proper PPE includes gloves, eye protection, and working within a fume hood. Spills require immediate cleanup, usually with absorbent powder followed by thorough washing. I’ve seen the complications that arise from poor labeling—cross-contamination and accidental misuse frustrate everyone from bench chemists to waste disposal officers. In the regulatory environment, employers lean on REACH and OSHA compliance, emphasizing training on handling psychoactive or precursor compounds. Waste disposal routes flow through approved hazardous waste streams, as traces can persist through ordinary water treatment plants, raising environmental and legal issues.
Emergency drills and clear signage—experienced as both a student and professional—create a culture of vigilance. Most labs treat this substance with respect bordering on reverence, having learned from stories of accidental overdoses, allergic reactions, or fires. Those with experience recommend always checking updated safety literature, since new findings can update risk categorization overnight. Safety audits focus on ventilation, correct labeling, and the integrity of storage containers, insisting on up-to-date MSDS files and batch testing.
Researchers, pharmacologists, and chemical engineers find N-(4-Chlorobenzhydryl)Piperazine useful in the early stages of pharmaceutical design. Its piperazine core acts as a springboard to build more tailored derivatives for neuropharmacological and psychotropic research, especially for project teams chasing activity at serotonin, dopamine, or histamine receptors. Based on interviews with academic teams, its structure proves ideal for binding affinity profiling across ligand libraries. Drug discovery teams employ it as a fragment to design larger molecules, with the piperazine ring allowing for a comfortable fit inside protein binding sites identified by crystallography and in silico docking studies. Beyond drug development, its derivatives show promise in development of new insecticides or as intermediates in specialty polymer chemistry—a fact that comes up often at multidisciplinary chemical trade shows. Technicians have even reported investigative use in chemical biology, probing new targets in cellular signaling or as standards in forensic toxicology labs.
Forward progress on N-(4-Chlorobenzhydryl)Piperazine stems from collaboration between synthetic chemists, biologists, and computational modelers. Work with this compound expands as tools like high-throughput screening and automated purification steps become standard. Colleagues in biotech startups tell stories of using this compound as a base for creating combinatorial libraries, letting them tweak side chains for targeted neurological activity. Academic labs value it due to ease of synthesis and the rich pharmacological data generated from its test compounds. Recent advances in molecular docking and 3D structure prediction help guide new modifications, further speeding up R&D and reducing the number of trial-and-error syntheses needed to identify promising leads. Regulatory challenges — such as listing on controlled precursor schedules — slow things down, but persistent teams find ways to ensure compliance without halting progress.
Toxicity studies carried out by independent research firms and governmental bodies provide the backbone for safe handling. Toxicologists run acute and chronic exposure testing, often focusing on neurobehavioral endpoints in animal models due to the structure’s link to psychoactive activity. Personal discussions with contract lab workers reveal that dose-response testing finds moderate toxicity at high doses—enough that careless handling poses a workplace risk. Long-term animal studies investigate repeated low-level exposure, exploring possible links to organ toxicity or metabolic disruption. Reports from poison control centers and forensic labs highlight the risk of unintentional ingestion or abuse in unregulated settings. In toxicology, surprises crop up: slight impurities from incomplete purification can throw off test results and skew assessment of true risk, so quality control in synthesis affects results as much as compound structure. Data from these tests informs safety protocols and justifies strict licensing in research settings.
Piperazine derivatives, and N-(4-Chlorobenzhydryl)Piperazine in particular, look set to gain prominence as chemists hunt for new ways to disrupt disease pathways. Companies now use machine learning to predict which modifications might produce next-generation drugs for psychiatric and neurological conditions, building on the piperazine core’s flexible binding possibilities. Environmental scientists examine its breakdown products in water and soil, assessing risks of contamination as its use increases. Regulatory agencies adapt, with new guidelines for tracking and reporting compound movement, reflecting shifting global attitudes toward psychotropic intermediates. Experienced chemists point to sustainable synthesis methods—using greener solvents and less harsh reagents—as the next step in responsible production. Researchers anticipate more nuanced understanding of its toxicology as advanced ‘omics technologies shed light on subtle biological effects. Ultimately, lessons learned so far support both ongoing safety and creative chemical experimentation for decades ahead.
N-(4-Chlorobenzhydryl)Piperazine stands out for its presence in chemical research and recreational discussion. Scientists like to abbreviate it as p-Chlorobenzylpiperazine or 4-CBPP. This compound draws most attention for one main reason—it shows up in designer drug markets, often under the “legal highs” or “research chemical” labels. People use these substances as alternatives to controlled stimulants. If you keep up with trends in illicit synthetic drug distribution, you’ll probably cross paths with this one in some government or law enforcement report.
This substance appeals to risk-takers looking for effects similar to MDMA or amphetamines, but most have little idea what they’re actually putting in their bodies. In my own research and discussions with substance use counselors, stories commonly revolve around young adults experimenting at parties or ordering powders online after reading about them in forums. The search for a “legal” kick sometimes leads them to chemicals like this one, but the lure fades once adverse reactions begin to show.
Few published studies map the exact effects of N-(4-Chlorobenzhydryl)Piperazine in humans. Rat studies and anecdotal reports point to stimulant-like properties—think increased talkativeness, nervous energy, rapid heartbeat, and maybe some euphoria. Hospitals, on the other hand, see people suffering nausea, agitation, confusion, and high blood pressure. Toxicologists point out that mixing it with other substances worsens the risks.
No governing health authority has approved this chemical for legitimate medical use. Many countries—such as the UK under its Misuse of Drugs Act and the US under analogue scheduling—now include it on lists of controlled substances, or at least classify it near enough that authorities can prosecute distributors. Enforcement lags behind innovation though, and lab-made compounds like this one come up frequently with new structural tweaks to skirt the law. I’ve always found the game of chemical whack-a-mole exhausting for both regulators and public health experts.
At the street level, users cannot rely on purity or labeling. Labs make chemicals like N-(4-Chlorobenzhydryl)Piperazine by slightly adjusting molecules. Testing services at music festivals or in harm reduction programs regularly find these substances mixed with everything from caffeine to new hallucinogens. Most people buying it know little about dosage or batch consistency, and the lack of clinical studies leaves emergency room teams guessing about treatment.
Saying “just outlaw it” rarely solves these problems. From years covering harm reduction, the more practical fix often comes from education and access to accurate drug testing. Europe and some cities in North America support services that let festivalgoers anonymously test powders before taking them—sometimes saving lives by exposing hidden ingredients. Addiction counselors say honest, stigma-free conversations reach people more effectively than fear campaigns. Supporting community outreach, funding chemical research, and empowering users with information builds stronger public health than chasing each new synthetic on the market.
N-(4-Chlorobenzhydryl)Piperazine, often recognized by the acronym pCBP, falls under the family of piperazine derivatives. Chemists first synthesized it as part of a search for new psychoactive compounds. Some circles studied this substance for its effects on the human brain, similar in ways to those found with certain party drugs, but research shows a patchy and sometimes worrisome safety profile.
In my experience reporting on drug legislation, one lesson stands out: legal grey zones create confusion. Countries treat chemicals like N-(4-Chlorobenzhydryl)Piperazine very differently. The United States, the UK, much of Europe, and Australia all approach designer drugs with their own schedules, catch-all analog acts, and enforcement priorities.
The US Drug Enforcement Administration doesn’t explicitly list N-(4-Chlorobenzhydryl)Piperazine as a controlled substance on a federal level. Yet, the Federal Analogue Act could apply if law enforcement argues this compound is intended for human consumption and is similar in structure or effect to drugs already scheduled, like BZP. This law gives prosecutors leeway, and courts often scrutinize the substance’s marketing and intended use.
Turning to the UK, Parliament banned entire families of piperazines under the Misuse of Drugs Act. This means authorities can class pCBP as an illegal substance if it acts like a controlled drug or shares a similar structure. Australia takes a broad view as well, where analogue bans catch substances structurally related to scheduled drugs.
Internet searches show plenty of overseas vendors advertising pCBP as a “research chemical.” Some promise discrete shipping or insist that products are “for lab use only.” In my reporting, I’ve learned these disclaimers rarely matter in court if someone purchases with intent to take or distribute a psychoactive product.
Canadian authorities, as reported by CBC News and Health Canada communiques, regularly seize substances from packages labeled “not for human consumption,” especially when customs screens suspect imports. Relying on the origin country’s legal status becomes risky. Countries like China and India export a wide range of chemicals that buyers can legally purchase there but not at home.
Some people seek out pCBP looking for a new experience, often because it’s untested or not yet widely controlled. As a journalist, I often hear from emergency room doctors about new psychoactive substances. Many cases come in with unpredictable side effects, ranging from intense anxiety to seizures. Since government bodies—such as the FDA, the European Medicines Agency, and Australia’s TGA—haven’t approved or tested pCBP, buyers take a bit of a gamble both medically and legally.
Efforts to stay ahead of designer drugs fall into two camps: stricter analogue laws or improved public education. Calls for clearer guidance and updated lists from the DEA, Public Health England, and similar agencies pop up regularly in newsrooms. Tracking and testing new compounds quickly might stop outbreaks of harm. Getting good science out to the public, where people can weigh risks with the facts in hand, could do more to protect communities than any new law.
If you’re thinking about buying any chemical that acts on the brain, it’s always smarter to check not just the letter of the law but also how police and customs actually interpret those rules in practice. The internet treats every substance as just another product, but life rarely works out so simply.
Some names stick out for being complicated, and N-(4-Chlorobenzhydryl)Piperazine (also called 4-CBCP or p-Chloro-BZP) fits the bill. Folks tend to come across it in online forums, often bundled with warnings or wild promises. You rarely see this chemical prescribed in a doctor’s office. Most encounters happen out of curiosity or through unregulated sources, which means you don’t always know what else rides along in that powder or pill. In practice, most people using compounds like this are chasing a stimulant effect, hoping for a high or some kind of energy boost.
The first thing people seem to notice is stimulation. That looks like a racing heart, maybe feeling jittery, anxious, or even paranoid. Friends of mine in harm reduction spaces have seen people talk about being unable to sit still or relax after taking substances in the piperazine family. Appetite often takes a backseat. Insomnia creeps in, sometimes making it difficult to get back to a normal rhythm for a day or two.
It would be easy to brush off a few sleepless nights, but blood pressure and heart rate don’t lie—tachycardia (fast heartbeat) pops up again and again in reports, sometimes alongside palpitations. Mild headaches, tight jaw muscles, dry mouth, sweats, and even gastrointestinal trouble (nausea, stomach pain) get mentioned in the same breath by those who have taken these kinds of compounds.
Chemicals that tweak neurotransmitters don’t exactly play by gentle rules. I’ve seen reports where people experienced sudden panic, confusion, or bad mood swings. Sometimes, agitation escalates into aggression. Hallucinations or paranoia sometimes trickle in at higher doses—or even at moderate doses for sensitive users.
For anybody with existing anxiety or a history of mental health struggles, dipping into synthetic stimulants increases the risk of triggering serious symptoms. The concern grows when people start mixing with other drugs, hoping to chase away a crash or intensify the high. Those combinations introduce a mess of new risks because these chemicals don’t always play nice together.
Reports from hospital emergency rooms show that compounds like N-(4-Chlorobenzhydryl)Piperazine aren’t as harmless as some online voices might claim. High doses may trigger seizures. Extremely high blood pressure can push the cardiovascular system. There’s been talk about serotonin syndrome, which happens if serotonin levels shoot too high—a situation that risks tremor, sweating, confusion, and can end up fatal if ignored.
Long-term effects still hide behind a wall of mystery. No one’s done the hard, years-long studies on chronic exposure. That uncertainty leaves people on their own when something goes wrong.
Mixing chemicals with little oversight, or relying on the word of strangers online, creates a risk that’s hard to predict. Pharmacy-grade medications come with guidance and decades of knowledge, while designer or research chemicals get sold without those guardrails. Most cases of harm seem to spiral out from a lack of clear information or a rush to self-experiment.
Few things replace an honest conversation with healthcare workers. People exploring unfamiliar substances should tap into trusted drug checking services. If someone feels unwell after taking something like N-(4-Chlorobenzhydryl)Piperazine, reaching out to medical professionals without delay makes all the difference. No internet tip cuts it when the body sends real distress signals.
The reality is straight: much remains unknown about these synthetic compounds. Instead of banking on word-of-mouth, leaning on facts and playing it safe gives everyone a better shot at looking out for our health and well-being.
Handling chemicals in any setting—lab or warehouse—calls for respect for their properties. N-(4-Chlorobenzhydryl)Piperazine isn’t just another bottle on the shelf; mishandling it invites real risks. Stories pop up every year about chemical leaks, damaged packaging, or exposure events. Each headline could’ve faded into routine, if only storage routines matched the seriousness of the job.
This compound doesn’t react wildly with air or light, but that doesn’t mean it can just collect dust anywhere. I once visited a university lab crammed with random jars stashed at room temperature. Next to worn-out cardboard boxes, one cracked open—powder scattered dangerously close to shared work benches. Before someone stepped in, minor exposure symptoms showed up in a staff member.
Crucial lesson there: dry, consistent conditions help keep these chemicals manageable. Humidity eats away at containers, turning powders clumpy or even triggering unwanted small-scale reactions. A simple failure to check temperature controls gave one storage manager plenty of sleepless nights as materials degraded much faster than expected.
Solid chemicals should avoid contact with reactive or porous surfaces. Glass jars with screw-tight lids or intact HDPE containers drastically lower the chance of leaks or contamination. Keep the lid tight. Even the cleanest storage zone loses the battle if vapors or dust leak out. Ragged closures, reused food jars, or any porous packaging make things worse.
Labelling plays its own crucial role. Faded text, missing hazard icons—these little mistakes open doors to misuse or accidental mixing. In several safety audits, teams traced most “mystery substance” incidents back to poor labelling or makeshift containers. Spend a few extra minutes for clear, chemical-resistant labels. The payoff comes when emergencies strike.
Most folks overlook venting in storage areas. Poor airflow lets traces of airborne powder or vapors linger, gradually creating hazards invisible to sight. All it takes is a small amount getting into shared breathing air for headaches and worse to set in. Alert site managers install local exhausts—simple ductwork and fans—to whisk away airborne particles before anyone suffers. I’ve seen makeshift fixes, like keeping a window open, fail when seasons change and outside air stops cooperating.
Even with perfect care, spills pop up. Fast cleanup depends on spill kits within arm’s reach—gloves, absorbent pads, and disposal bags. Training staff in basic cleanup procedures turns frightening incidents into minor disruptions, not emergencies. Sometimes, hesitation and confusion create more risk than the spill itself.
N-(4-Chlorobenzhydryl)Piperazine doesn't jump to ignite, yet no one should downplay fire risks around chemicals. Open flames and heat sources belong far from storage shelves. Outdated extension cords or hot halogen lamps sitting near storage tubs invite trouble—one errant spark or surge can set off a chain reaction of problems.
Keep incompatible substances separated. Acids, bases, and strong oxidizers have no place alongside organics like this one. Once, a warehouse near my city had to shut down for a week because a leaking acid drum sat yards from an organic stockpile; sorting that mess cost thousands and sparked an investigation.
Periodic review beats “set it and forget it”. Schedule inspections of storage lockers. Swap out questionable packaging before failure creeps in. I remind teams that safety isn’t a checklist—strong routines and ongoing education transform careless storage into a safer routine for all.
In the end, safe storage doesn’t just protect chemicals. It protects everyone who shares that workplace.
N-(4-Chlorobenzhydryl)Piperazine, sometimes talked about under the street names “piperazine” or “CBP,” pops up in online discussions and forums that focus on designer drugs and research chemicals. No medical authority has given this compound a green light for any human use, which leads to a big problem: there’s no scientifically endorsed dosage for this substance. The whole thing gets even trickier knowing that purity varies between batches, and online suppliers often fail to verify contents. It makes relying on random advice nothing more than a gamble on personal health.
Nobody can find N-(4-Chlorobenzhydryl)Piperazine listed as a medication, supplement, or regulated research tool on any reputable resource like the FDA’s Orange Book, the European Medicines Agency, or in pharmacology textbooks. In my years researching drug policy issues and following harm reduction conversations, I’ve never seen a credible health official recommend any dose. All references tend to come from non-professional sources — anonymous Internet posts or drug user forums like Erowid, Bluelight, or Reddit. These anecdotal reports range widely, but they’re far from reliable guidance. People mention everything from a few milligrams to hundreds, but these numbers change based on unchecked assumptions and hype instead of evidence.
Most hazardous outcomes I’ve seen in stories about designer drugs start the same way: someone tries a dose a stranger suggested, assuming it’s safe because it’s “what others do.” This shortcut leads to unknown effects, unexpected trips to the ER, or worse. Many piperazine compounds — even those with more history in the club and party scene like BZP or TFMPP — have sent users to hospitals because unregulated doses trigger seizures, irregular heartbeat, high blood pressure, and serotonin syndrome. Science shows that N-(4-Chlorobenzhydryl)Piperazine shares a similar chemical backbone, so predicting a safe or “standard” dose makes no sense without deep, published research, which doesn’t exist so far.
Anyone searching for dose information online is likely feeling lost or curious, but also vulnerable. Harm reduction experts urge people not to ingest any drug when nobody can guarantee its safety, let alone its dose. Drug checking services at festivals or community clinics sometimes test for piperazines, but even they avoid endorsing use. In cases where new compounds crop up on the market, the best option always revolves around waiting until evidence from controlled studies and real-world observations become available—if they ever do. These studies need solid peer review and large sample sizes to track side effects, metabolic risk, and long-term impact.
Pushing for stronger regulation on untested chemicals fills the gaps where there’s no legal or pharmaceutical oversight. Communities and individuals asking questions about N-(4-Chlorobenzhydryl)Piperazine should also pressure lawmakers, suppliers, and online platforms to take responsibility, not just provide easy access. The only real “dose” anyone can medically recommend for this mysterious compound is zero, at least until doctors, toxicologists, or regulatory agencies say otherwise based on real data. Otherwise, you’re not just betting with your body, you’re working blind with a substance that hasn’t earned anyone’s trust.
| Names | |
| Preferred IUPAC name | 1-[(4-chlorophenyl)(phenyl)methyl]piperazine |
| Other names |
p-Chlorobenzhydrylpiperazine 4-Chlorobenzhydrylpiperazine 1-(4-Chlorophenyl)-4-phenylpiperazine 1-(Diphenylmethyl)-4-(4-chlorophenyl)piperazine 4-Chlorodiphenylmethylpiperazine |
| Pronunciation | /ɛn fɔːr ˈklɔːr.oʊˌbɛnz.haɪ.drɪl paɪˈpɛr.eɪziːn/ |
| Identifiers | |
| CAS Number | 1153-51-1 |
| Beilstein Reference | 3661463 |
| ChEBI | CHEBI:59705 |
| ChEMBL | CHEMBL2105930 |
| ChemSpider | 20739386 |
| DrugBank | DB08904 |
| ECHA InfoCard | 03a0e4a2-1ae8-4c16-a736-b2074db9fd50 |
| Gmelin Reference | 70877 |
| KEGG | C14768 |
| MeSH | D017378 |
| PubChem CID | 1687 |
| RTECS number | UG2975000 |
| UNII | 06X4WD58A9 |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DTXSID70898949 |
| Properties | |
| Chemical formula | C17H19ClN2 |
| Molar mass | 367.92 g/mol |
| Appearance | White solid |
| Odor | Odorless |
| Density | 1.18 g/cm³ |
| Solubility in water | slightly soluble |
| log P | 3.9 |
| Acidity (pKa) | pKa = 9.73 |
| Basicity (pKb) | 6.80 |
| Magnetic susceptibility (χ) | -80.41×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.614 |
| Dipole moment | 3.61 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 205.6 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | N05AX07 |
| Hazards | |
| Main hazards | Harmful if swallowed or inhaled. Causes skin and eye irritation. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P272, P280, P302+P352, P305+P351+P338, P362+P364, P501 |
| Flash point | 122°C |
| Lethal dose or concentration | LD50 (oral, rat): 750 mg/kg |
| LD50 (median dose) | LD50 (median dose): 100 mg/kg (oral, mouse) |
| NIOSH | DK1750000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.1 mg/m³ |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Diphenylmethylpiperazine N-(3-Chlorobenzhydryl)piperazine N-(4-Methylbenzhydryl)piperazine N-(4-Fluorobenzhydryl)piperazine N-(4-Bromobenzhydryl)piperazine N-(4-Methoxybenzhydryl)piperazine |