The march of synthetic chemistry brought N-(2-Hydroxyethyl)-Piperidine to the wider scientific stage in the mid-20th century. Researchers searching for robust, adaptable amine building blocks kept circling back to the piperidine ring and its derivatives. Once industrial solvents and surfactants became more widespread, chemists leaned on hydroxyalkyl substituents, recognizing their impact on solubility and reactivity. N-(2-Hydroxyethyl)-Piperidine sits among these meaningful innovations—a single modification giving rise to improved application in organic synthesis, pharmaceuticals, and specialty formulations. My first encounter with this compound came in a lab testing corrosion inhibitors for ethanol-blended fuels; the inclusion of the hydroxyethyl group opened creative routes in formulation not possible with simpler piperidines.
N-(2-Hydroxyethyl)-Piperidine appears as a clear, nearly colorless liquid, often with a faint ammoniacal odor. The molecular structure features a six-membered piperidine ring with a two-carbon hydroxyethyl group attached to the nitrogen, increasing both hydrophilicity and flexibility. The boiling point generally lands in the range of 235–240°C at standard pressure, making it suitable for high-temperature processing where other amines might react or volatilize prematurely. The melting point rests well below room temperature, and the chemical dissolves with ease in water, ethanol, methanol, and many polar organic solvents. The basic nitrogen draws attention during acid-base titrations, and the presence of both amine and hydroxyl functionalities allows this molecule to act as both nucleophile and hydrogen-bonding participant, qualities valued by formulation chemists.
Producers usually offer N-(2-Hydroxyethyl)-Piperidine in technical and high-purity grades. Typical specifications might set purity above 98%, with water content controlled below 0.5% and traces of piperidine or dihydroxyethylamine monitored by gas chromatography. Packaging often reflects demands of both safety and efficiency: steel drums with inert inner linings for volume buyers, high-density polyethylene bottles for lab supply chains. Safety labeling follows established protocols such as GHS, highlighting possible risks including skin irritation and eye damage, and outlining response measures. Since it's classified as a secondary amine alcohol, proper container labeling and documentation are key for all handlers along the supply chain.
Industrial production usually involves the reaction of piperidine with ethylene oxide, managed under basic or neutral conditions to suppress side reactions. Batch reactors dominate this segment, with precise temperature control crucial for yield and product quality. I once observed a plant manager wrangling with exothermic excursions during scale-up, as the fine balance between conversion efficiency and impurity formation tests even experienced operators. Post-reaction workup routines almost always rely on neutralization, distillation, and vacuum stripping to purify the product. While continuous processes exist, batch synthesis remains favored where flexibility in product tailoring or small-lot, high-purity output commands a premium.
Versatility comes from its dual functionality: the piperidine nitrogen and hydroxyethyl tail offer sites for further functionalization and salt formation. Typical reactions include esterification with acyl chlorides to yield hydroxyesters, N-alkylation or acylation, and oxidation of the alcohol group to aldehydes or acids. In organic synthesis, the hydroxyethyl group stabilizes intermediates or acts as a chelating agent in catalysis. In my hands, reactions with diisocyanates produced flexible polyurethanes with enhanced wetting properties—an application that kept a customer’s coatings line running smooth in high-humidity seasons.
Chemical catalogues and regulatory databases often list this compound under names such as 1-(2-Hydroxyethyl)piperidine, N-Hydroxyethylpiperidine, or 2-(Piperidin-1-yl)ethanol, reflecting IUPAC and common naming conventions. Brand names and trade names occasionally emerge among specialty chemical suppliers, sometimes tied to their proprietary processes or market ambitions. Knowing alternative labels helps cross-verify regulatory documents, which keeps a project on track, especially when sourcing internationally.
Working around N-(2-Hydroxyethyl)-Piperidine means respecting its chemical reactivity and acute toxicity risks. Direct contact sometimes provokes skin or eye irritation, so gloves, goggles, and ventilation aren’t negotiable. Since the compound can form nitrosamines—recognized carcinogens—when combined with nitrite-containing chemicals, facilities follow strict segregation and cleaning protocols. Local exhaust ventilation, routine surface monitoring, and employee health checks help keep exposure below occupational limits. Companies invest in spill kits and first aid resources to address accidental release or contact. Waste handling spells out discharge concentrations and needs for incineration or controlled biodegradation, given environmental persistence factors.
N-(2-Hydroxyethyl)-Piperidine keeps finding new homes in modern industry. Waterborne coatings, textile softeners, pharmaceutical intermediates, corrosion inhibitors, and lubricants each tap into the capabilities offered by its alkylamide structure. Drug chemists access its ring as a handle for scaffolding, while polymer scientists incorporate the hydroxyethyl function to boost plastic flexibility and dyeability. Niche uses pop up in agriculture, where tertiary amines serve as adjuvants or compatibilizers in herbicide blends. Across these sectors, engineers value reliable sourcing, tightly defined specifications, and strong documentation to safeguard process consistency and product safety.
Academic and industrial R&D labs continue to expand the footprint of N-(2-Hydroxyethyl)-Piperidine by leveraging its bifunctional nature. In medicinal chemistry, efforts target the synthesis of antipsychotic, antiviral, or antihypertensive lead compounds by decorating the piperidine core with additional pharmacophores. Materials scientists use it to engineer hydrophilic/hydrophobic interfaces in amphiphilic polymers. As green chemistry standards evolve, researchers scrutinize process innovations aiming to cut solvents, reduce by-products, and improve energy use in both small- and large-scale operations.
Toxicological studies show moderate acute toxicity from oral and dermal routes, raising attention from safety regulators. No single report wraps up its risk profile; studies rely on animal models and occupational health outcomes, focusing on skin irritation, potential organ toxicity, and long-term exposure to vapor or mists. Nitrosamine formation deepens concern, as incidental exposure in high-temperature or acidic conditions may trigger risk. Proper handling, PPE, and workplace air monitoring keep users within safety margins. Reading published data helps chemists and industrial hygienists develop clearer risk assessments, informing both design and operational planning.
Strong demand continues in established markets as N-(2-Hydroxyethyl)-Piperidine supports new molecular designs in medicines, smart polymers, and high-performance additives. Increased regulatory scrutiny—particularly around nitrosamine potential and environmental fate—pushes producers to tighten purity controls and innovate greener synthesis pathways. Forward-looking projects focus on biodegradable derivatives and renewable feedstocks, reflecting the sustainability imperative shaping every chemical sector. Recent research projects promise to open new avenues in biomedicine, catalysis, and environmental remediation, confirming the lasting relevance of this underappreciated molecule.
Talking about N-(2-Hydroxyethyl)-Piperidine means looking beyond the mouthful of a name and zeroing in on its atoms. The formula for this compound is C7H15NO. To break that down, we see it holds seven carbon atoms, fifteen hydrogen atoms, a single nitrogen atom, and an oxygen atom. There’s a lot going on in this small corner of organic chemistry, even if it’s not a headline on the nightly news.
I’ve seen chemicals grouped into broad categories, but the formula shapes what a substance does and how it interacts. C7H15NO represents a molecule with a piperidine ring attached to a hydroxyethyl group through that nitrogen atom. In the lab, every letter and number counts. You could be developing a pharmaceutical intermediate, working on a new cleaning agent, or studying nervous system models. Small changes in structure can flip safety, use, or legal standing.
That detail about a hydroxyethyl group may feel tiny. Still, as a researcher, I remember how that single extra –OH changes water solubility and how a compound sticks to other molecules. N-(2-Hydroxyethyl)-Piperidine is a good example of why connections between atoms drive results. It’s not just a piperidine ring floating in space. That side group brings new opportunities for chemical reactions or drug design.
Specialty chemicals often show up behind the scenes in factories and research labs. You may run across C7H15NO when synthesizing advanced polymers, adjusting pH stability, or preparing solvents for extractions. People who work with these chemicals rely on knowing exactly what’s in the bottle or drum. It’s not about memorizing formulas—it’s about safety and function. Mishandling the wrong compound because of a mix-up in names or formulas has real consequences.
Errors sneak in easily. I’ve seen a typo in a formula cause hours of confusion in student labs, and it only takes a missed hydrogen atom for a process to break. N-(2-Hydroxyethyl)-Piperidine highlights the need for clear communication and a good handle on both naming conventions and molecular structure. Spelling out the formula forces people to rethink assumptions and double-check their work. That pays off on the bench and in industry.
An accurate formula doesn’t come just from trust in a supplier or memory from a textbook. Real value shows up through verification. Tools such as NMR, IR spectroscopy, or mass spectrometry give confirmation and peace of mind. In my experience, teams that spend a few extra minutes checking their formulas run smoother projects with fewer mistakes and mishaps.
Another path toward reliable results comes from better training. Digging into why that C7H15NO actually means N-(2-Hydroxyethyl)-Piperidine closes the gap between rote memorization and real understanding. There’s still room for improvement in chemical safety training, particularly for those new in labs or handling intermediates. Laying out the structure, not just the formula, connects abstract chemistry to practical work.
Most folks outside the lab might never think about N-(2-Hydroxyethyl)-Piperidine. For those working with specialty chemicals, knowing details like C7H15NO is part of daily life. Getting those details right is never just an academic exercise. It’s about reducing mistakes, protecting workers, and making real advances in products people use every day.
N-(2-Hydroxyethyl)-Piperidine doesn’t show up in most headlines, but it stays busy behind the scenes in several big sectors. In coatings, paints, and polymers, it pulls double duty as a building block that helps manufacturers shape the physical and chemical properties of finished products. Its structure lets chemists tweak hardening time and resistance to water or abrasion, solving some very real problems faced in manufacturing and maintenance.
Human health keeps demanding new and better therapies, and chemists keep hunting for tools to tackle stubborn problems. Here, N-(2-Hydroxyethyl)-Piperidine serves as a handy intermediate, especially during the synthesis of drugs targeting neurological and cardiovascular disorders. Its molecular arrangement allows for fine-tuned alterations, which can mean safer, more effective drugs. This flexibility draws attention from research teams working to outsmart infectious diseases or chronic illnesses.
Steel pipelines, ships, and bridges take a beating from air, water, and chemicals. Corrosion eats through assets and budgets. N-(2-Hydroxyethyl)-Piperidine steps in as part of lubricant and anti-corrosive formulations, extending operational life and saving companies real money on repairs and downtime. Oil and gas outfits see the value because shutdowns and leaks aren’t just disruptions—they cost workers' safety and millions in lost revenue.
Farmers count on products that help keep crops healthy while minimizing environmental fallout. N-(2-Hydroxyethyl)-Piperidine offers a foundation for several agrochemical agents. Chemists can make pest control products that stick better to plant surfaces, break down more safely, or hit target pests harder. In home care or cleaning agents, this compound helps blend actives more evenly, so consumers get bottles that work from the first spray to the last drop.
Modern chemical use doesn’t escape attention. People want products that score high on safety and tread lightly on the earth. Companies using N-(2-Hydroxyethyl)-Piperidine have faced mounting questions about hazards, waste, and sourcing. Handling this compound carefully matters because improper storage or disposal can have real consequences. It takes investment in employee training and responsible supply chains to minimize exposures and keep work environments safe.
Researchers and industrial leaders keep working on greener processes. One promising direction: switching to renewable feedstocks and updating manufacturing techniques to cut toxic byproducts. Industry groups have started collaborating on life-cycle assessments, which put hard numbers behind claims of safety and sustainability. Governments and watchdogs push for clear labeling and better tracking—a move that builds trust and lets everyone make informed choices.
N-(2-Hydroxyethyl)-Piperidine may not turn up in daily conversation, but its influence shows up in safer medicines, sturdier structures, cleaner homes, and fields yielding enough to feed growing populations. Its proper use gets linked to savings, efficiency, and better health—all worth the investment in responsible practices that don’t risk tomorrow for quick gains today.
I’ve worked in labs where chemicals with odd names like N-(2-Hydroxyethyl)-Piperidine sound innocent, but open a bottle and the story shifts. This compound carries irritant qualities. Breathing its vapors or letting it linger on skin makes for a rough day. Even without a chemistry degree, common sense says this stuff does not belong near bare hands, open eyes, or a sandwich.
N-(2-Hydroxyethyl)-Piperidine won’t start fires on its own, yet it reacts with strong acids and oxidizers. Mixing chemicals without triple-checking the label never ends well. In 2020, a research group in Germany traced minor lab injuries to careless mixing. So it’s always smart to treat even seemingly minor reactives with respect.
A splash of this substance on bare skin stings and often takes more than a simple rinse to forget—gloves matter. I use nitrile gloves, checked for holes, before unscrewing the cap. Regular latex breaks down faster. Eyes need real goggles, not just reading glasses. Once, I saw a student rubbing an irritated eye after a tiny splash. He ended up at the clinic. Goggles sit between a safe day and a hospital visit.
Open-toed shoes belong far away from any workspace with this chemical. Closed shoes and a lab coat block random spills from spreading. Fume hoods aren’t just glossy, expensive cabinets; they clear the air of vapors you don’t see or smell at first. Not every lab has five-star ventilation. Turn the hood on, and keep containers closed tightly unless actively pouring.
Good safety begins long before measuring anything. Every bottle should bear a clear, printed label showing the chemical, hazard information, and the date opened—no scribbled initials. Storage goes on a stable shelf, locked away from strong bases, acids, or oxidizers. Even a tiny mix-up can start an unwanted reaction.
In my own experience, disorganized shelves invite small disasters. Clear labels, physical separation, and dated inventory sheets reduce confusion and the urge to grab the wrong bottle in a hurry. The Chemical Safety Board recommends annual checks, and I’ve seen firsthand how these reviews catch aging bottles or faded warnings before trouble starts.
Pouring leftovers down the drain does not save time; it turns water treatment into a guessing game. Used N-(2-Hydroxyethyl)-Piperidine belongs in labeled waste containers. Each university or workplace sets its own disposal schedule, and skipping steps risks fines or worse. If you feel like rushing, remember a true story from my city, where improper disposal ended with a blocked drain and a pricy cleanup.
Wash hands carefully with soap and water after working, even with gloves. Contamination does not always look obvious, and the habit stops chemicals from getting to your coffee or phone.
OSHA and other agencies push for yearly safety training, but the best habits come from experience and reminders. I’ve been in sessions where sharing close calls and mistakes made the rules stick more than memorizing them. Ask questions if unsure. The only bad question is the one that goes unasked.
Handle N-(2-Hydroxyethyl)-Piperidine as if today’s small step shapes tomorrow’s safety. Smart handling, clean work habits, protective gear, and a culture of speaking up turn chemical risk into manageable routine.
Looking at N-(2-Hydroxyethyl)-piperidine, it’s clear this chemical isn’t just another basic ingredient on a shelf. Labs and manufacturers use it for a stack of processes, including organic synthesis and pharmaceutical research. Because of this, its safe handling and storage have a direct impact on workplace safety and product stability. In my experience, even a short lapse in chemical care can lead to unnecessary health risks or expensive product loss.
This compound stands up well in controlled environments, but swings in temperature or carelessness make it unpredictable. Most reputable chemical suppliers note that N-(2-Hydroxyethyl)-piperidine performs best at room temperature—too much heat speeds up breakdown or causes pressure build-up in sealed containers. In a busy storage room, I’ve seen labels curl and containers deform just from being kept close to a hot water pipe.
Direct sunlight poses a hazard, too. Windows can turn a shelf into a slow cooker, degrading sensitive chemicals. Simple steps like storing containers away from light and in a spot with little temperature fluctuation can lengthen shelf life. Humidity also plays a part; exposure to moisture endangers both the purity of the chemical and the safety of workers.
Precision matters most with chemicals. Using tightly sealed, clearly labeled containers helps keep moisture and air out. Over the years, I’ve run into fewer headaches by double-checking that lids screw tight and that shelving sits stable. Keep N-(2-Hydroxyethyl)-piperidine in glass or compatible plastic—metal sometimes reacts and warps, risking unwanted changes in the material or dangerous leaks.
Labeling should not be underestimated. Use water-resistant markers and labels since spills and splashes happen. This isn’t just good housekeeping; regulations in many countries actually demand it. Following these standards massively cuts potential confusion when rotating stock or responding in an emergency.
In my own lab days, keeping reactive chemicals apart saved countless headaches. N-(2-Hydroxyethyl)-piperidine reacts in odd ways with acids, oxidizers, or even strong bases. Tucking it away on a dedicated shelf, away from these potential troublemakers, reduces the chance of a hazardous event.
A no-food-or-drink policy near chemical storage sounds basic but reminders help. Even careful professionals sometimes use break rooms for overflow storage after a delivery. This creates contamination risks for both chemicals and food—an easy one to avoid by maintaining clear separation.
Fire safety looms large in storage planning. N-(2-Hydroxyethyl)-piperidine itself doesn’t burn eagerly, but surrounding combustible materials can turn a small issue large. Having spill kits, eyewash stations, and fire extinguishers in place is more than just ticking a box. In my work, reviewing emergency pacing during drills often uncovered issues in storage layout that wouldn’t be obvious day to day.
Routine checks ensure that containers stay sealed, labels stay readable, and chemicals stay upright and undisturbed. This straightforward attention to detail prevents small leaks or accidental exposure. Regular inventory turns up expired or degraded stock fast, trimming the chance of accidental use.
N-(2-Hydroxyethyl)-piperidine rewards careful storage with stable shelf life and safer handling. Even quick wins—like placing chemicals away from sunlight and heat, sealing containers, and training staff—go a long way. Every workplace can benefit from a tight focus on chemical safety. There’s no substitute for hands-on attention to where and how chemicals sit, not just for regulatory compliance, but for everyone’s peace of mind.
N-(2-Hydroxyethyl)-Piperidine’s not exactly the sort of compound you’ll see at a local store. Folks usually bump into it working in chemical synthesis, pharmaceuticals, or research labs. Its structure—a piperidine ring hooked to a hydroxyethyl group—finds uses making specialized chemicals, certain drugs, and refining industrial products.
Plenty of online sellers claim to offer nearly every reagent or compound under the sun. Sometimes, these sites pop up overnight, promising quick shipping and “lab grade” quality with no paperwork. Whenever I need a specialty chemical like N-(2-Hydroxyethyl)-Piperidine, the first thing I check is the supplier’s credentials. Trusted suppliers, the names researchers recognize—MilliporeSigma, Alfa Aesar, TCI America—back up their listings with technical data, safety documents, and real customer service. Real distributors require proof of legitimate use, not just a credit card. There’s a reason for that: chemical safety laws keep both buyers and communities out of trouble.
A compound like N-(2-Hydroxyethyl)-Piperidine isn’t nearly as innocent as baking yeast. Handling it without training can bring on chemical burns, toxic fumes, or worse if it ends up misused. Stories of accidents or illegal diversions scroll across industry headlines all the time. For experienced professionals, strict protocols for transport and storage aren’t just a rule, they’re a way of staying healthy. From my own work with similar chemicals, chemical-resistant gloves and well-ventilated hoods become second nature after witnessing just one mix-up.
In the U.S. and Europe, rules steer who buys and stores compounds like this. Companies check identification and verify business licenses. Importing rare chemicals without following federal rules can mean facing huge fines. Some compounds have extra restrictions if they could be used for illicit synthesis. Chinese and Indian suppliers often list these chemicals more openly, but international shipping brings customs inspections and the risk of seizure unless paperwork’s airtight. I’ve watched shipments get delayed for weeks because just one form from a university lab didn’t line up right with what customs wanted.
If the goal is a scientific experiment, reaching out to a university or larger company with a chemical buying program could avoid headaches. Many scientists order through centralized procurement, which cuts down the risk of counterfeit products or regulatory snags. Hobbyists or amateurs looking for specialty chemicals often run into brick walls—for good reasons. If the compound’s for a home project or personal use, it’s worth rethinking whether another, safer chemical could fit the bill.
Every year, chemical safety rules evolve, especially as new risks come to light. In my own lab circles, keeping up with changing lists from the Drug Enforcement Administration or the Environmental Protection Agency isn’t a chore; it’s a must. Any project involving unusual reagents starts with research, includes consulting with trained professionals, and finishes only after confirming the order’s legal. No shortcut beats staying informed, not just for safety’s sake, but for keeping projects from grinding to a halt over avoidable mistakes.
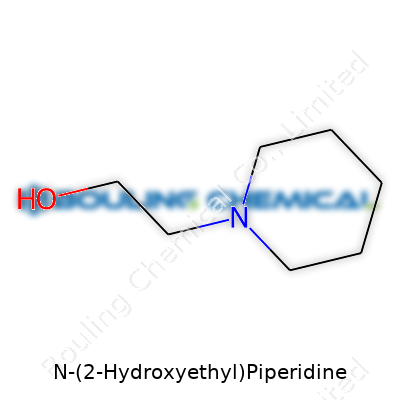
| Names | |
| Preferred IUPAC name | 2-(Piperidin-1-yl)ethan-1-ol |
| Other names |
HEP 2-(Piperidin-1-yl)ethanol N-Hydroxyethylpiperidine Piperidine, N-(2-hydroxyethyl)- |
| Pronunciation | /ɛn tuː haɪˈdrɒksiˌɛθɪl paɪˈpɛrɪdiːn/ |
| Identifiers | |
| CAS Number | 111-41-1 |
| 3D model (JSmol) | `CCCCN(CCO)CC1CCCCC1` |
| Beilstein Reference | 1452301 |
| ChEBI | CHEBI:131741 |
| ChEMBL | CHEMBL3305799 |
| ChemSpider | 37319 |
| DrugBank | DB08282 |
| ECHA InfoCard | 100.011.014 |
| EC Number | 203-057-1 |
| Gmelin Reference | 142262 |
| KEGG | C06046 |
| MeSH | D010883 |
| PubChem CID | 8717 |
| RTECS number | TL9275000 |
| UNII | TQ0462C89Z |
| UN number | UN2735 |
| CompTox Dashboard (EPA) | DTXSID5052787 |
| Properties | |
| Chemical formula | C7H15NO |
| Molar mass | 129.21 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | amine-like |
| Density | 0.997 g/cm3 |
| Solubility in water | Soluble |
| log P | 0.02 |
| Vapor pressure | 0.02 mmHg (25°C) |
| Acidity (pKa) | 9.66 |
| Basicity (pKb) | 5.94 |
| Magnetic susceptibility (χ) | -62.32·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.484 |
| Viscosity | 14 cP (20°C) |
| Dipole moment | 3.07 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 322.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -340.3 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | –4277 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | N07BC03 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H312, H314 |
| Precautionary statements | P264, P280, P302+P352, P305+P351+P338, P362+P364, P332+P313 |
| NFPA 704 (fire diamond) | 1-3-0 |
| Flash point | 100°C |
| Autoignition temperature | 340°C |
| Explosive limits | Explosive limits: 1.1–6.4% |
| Lethal dose or concentration | LD50 (oral, rat): 530 mg/kg |
| LD50 (median dose) | LD50 (median dose): 460 mg/kg (rat, oral) |
| NIOSH | SN8900000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.1 ppm |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Piperidine 2-Hydroxyethylamine (Ethanolamine) N-Methylpiperidine N-Ethylpiperidine N-(2-Hydroxyethyl)morpholine N-(2-Chloroethyl)piperidine Piperazine Diethanolamine |