Digging back into the history of Morpholinium Toluene-4-Sulphonate, research shows steady progress that echoes broader synthetic pathways in organosulfur chemistry. Around the 1970s, this compound gained attention as chemists sought robust alternatives for ion exchange and catalysis. Back then, focus centered on how to maneuver between stability and reactivity for morpholinium salts. Laboratories looked to blend morpholine’s ring structure, valued for its balance of hydrophilicity and resistance to degradation, with sulfonated aromatics, taking cues from the long industrial legacy of toluene derivatives. Over decades, synthesis methods tightened up, waste streams dropped, and yields crept higher, with each improvement driven by hands-on experience in both academic and industrial settings.
Morpholinium Toluene-4-Sulphonate lines up as a crystalline salt, shaped by the pairing of morpholinium cations with para-toluenesulphonate anions. As an intermediate, it presents a way to harness morpholine’s base strength while relying on the thermal and chemical durability that aromatic sulphonates bring. Bench chemists working on scale-up find the compound’s shelf stability and predictable melting range eliminate plenty of headaches—fewer surprises means smoother workups and less downtime.
Observing a sample of this material, one notes its white to off-white crystalline or powdery appearance. With a melting point typically nestled between 175°C and 185°C, it avoids the stickiness that plagues some other organic salts. The compound dissolves easily in polar solvents like water or methanol and resists decomposition up to fairly high temperatures. On the chemical side, both the morpholinium ion and sulphonate anion remain chemically distinct, neither masking nor dulling the other’s reactivity during mixing or reaction. This two-part character lets researchers dial in parameters and shift functions based on reaction goals.
Product integrity stays front and center for any application. Quality control teams often screen for purity at 99% and above, specifying water content below 1%. Any reputable supplier should clearly label for gross and net mass, batch number, date of manufacture, and recommended storage temperatures—typically cool, dry, away from acids or oxidizers. In my experience, accurate specifications speed up regulatory review and grant certainty to downstream users.
Crafting Morpholinium Toluene-4-Sulphonate starts with a simple acid-base reaction: morpholine meets para-toluenesulphonic acid, often in an aqueous medium. Begin with a cold-water slurry of sulfonic acid and add morpholine dropwise; gentle stirring manages exothermicity, preventing runaway heat or local degradation. Post addition, letting the mixture stand or applying mild heat crystallizes the salt. Filtration, thorough washing with cold water, and drying under vacuum finish the process. Direct, stepwise prep such as this limits byproducts, keeps purification straightforward, and supports batch reproducibility on both lab and plant scales.
Morpholinium Toluene-4-Sulphonate serves as more than just an inert salt. Swap in this material during alkylations, acylations, or even Mannich-type reactions to moderate pH or buffer sensitive intermediates. It finds roles in phase transfer catalysis, where its dual ionic nature allows solubility in organic and aqueous phases. For researchers wanting to tweak properties, modification routes include N-alkylation of the morpholinium ring or substitution at the toluene para position before salt formation. Every change brings a suite of altered solubility, melting, and conductivity traits—tools for tuning processes with data-backed confidence.
Over the years, the literature records this salt under quite a few names: 4-Methylbenzenesulfonic acid morpholinium salt, Morpholine p-toluenesulphonate, and Morpholinium tosylate count among the most charted. Some catalogs use the CAS registry number, others prefer systematics like N-Morpholine-p-toluenesulfonic acid salt. Trade names occasionally pop up, especially when linked to specific grades or proprietary formulations, but core chemical identity never gets lost in the translation.
Standardization of safety practices makes a real difference for anyone handling morpholinium salts. Contact with skin or eyes should be avoided, since the sulfonate moiety can irritate mucous membranes. Gloves, safety goggles, and lab coats form the basic barrier, while fume hoods cut down on inhalation exposure during reaction setup or scale-up. Material Safety Data Sheets (SDS) point to dust control as a priority, since powder forms disperse easily. Storage in double-sealed containers, out of reach of minors and away from incompatible agents, insulates against accidental spills or cross-contamination. Regular review of protocols ensures alignment with evolving occupational health standards and regulatory mandates.
Chemists lean on Morpholinium Toluene-4-Sulphonate for its ability to balance reactivity and control in synthesis. In pharmaceuticals, the salt stabilizes intermediates during key steps, acting as a buffer without leaving unwanted residues. Sectors focusing on electroplating and catalysis exploit its ionic conduction and long-term chemical durability. Polymerization reactions sometimes feature this salt as a catalyst or phase transfer agent, drawing on its easy dispersibility and anchoring influence over reaction rates. During my time in the lab, working to optimize dye intermediates or specialty coatings, we relied on its steady performance to pull out improved yields and cleaner products, even under demanding conditions.
R&D teams test this compound for emerging uses in fine chemical production, green chemistry, and next-gen battery development. Analytical chemists experiment with salt modifications, aiming for enhanced selectivity in catalytic cycles or improved environmental compatibility. Some research groups focus on converting waste sulfonates from other industrial streams by linking them to morpholine bases, finding synergy in sustainability and scalability. My exchanges with university colleagues confirm steady funding and diverse inquiry—from bioactive molecule synthesis to deep eutectic solvents. Each iteration builds new knowledge on top of a strong technical foundation.
Toxicologists approach morpholinium salts by mapping both immediate and chronic effects. Acute toxicity presents as mild to moderate for skin and mucous contact, aligning with data from closely related morpholine and sulfonate structures. Long-term animal studies indicate low systemic toxicity, yet routine inhalation or ingestion remains unadvised. Researchers keep pursuing in vitro and in vivo studies on metabolic breakdown and persistence, especially following environmental release or accidental exposure. Regulatory risk assessments incorporate those findings into workplace exposure limits, fire and explosion hazard ratings, and environmental safety reports. During hazardous material audits, clear labeling and accident response play essential roles in preventing negative outcomes.
Given the movement toward greener synthesis, Morpholinium Toluene-4-Sulphonate stands out as a versatile intermediate. Industry watchers look at its future not just as a buffer or ion exchanger, but as a platform for smart solvents, nano-structured catalysts, and drug conjugates. More projects target optimization of its synthesis to prioritize minimization of waste, use of renewable precursors, and improved atom economy. Advanced digital modeling promises to reveal new substitution patterns or reaction applications that were tough to access in the past. My direct work with chemical development leads me to expect broader use in interdisciplinary sectors—where clear specifications, robust safety frameworks, and deep technical know-how will determine the pace of progress.
Morpholinium toluene-4-sulphonate, a mouthful of a name, tends to get noticed more in specialty chemical circles than in everyday households. My own introduction to this compound happened through a research lab setting, surrounded by engineers debating how to make materials smarter and more resilient. It turns out that substances like morpholinium toluene-4-sulphonate often appear where innovation demands fine-tuned chemistry, especially in solvents, coatings, inks, and electronics manufacturing.
This salt usually acts as an ionic liquid—a liquid at room temperature that conducts electricity. Unlike typical table salt, morpholinium toluene-4-sulphonate is less about sprinkling and more about enabling. In resin formulations, this chemical can enhance the conductivity of electrolytes in batteries, or push paints and varnishes to dry quicker on surfaces. In some specialty inks, it allows printers to run at high speeds without smearing, which matters to anyone frustrated by a messy label or an unreadable barcode.
Researchers in the renewable energy space often turn to compounds like these to tweak the performance of solar cells. Cost remains a constant barrier in this field, and chemical tweaks can drive efficiency up just enough to tip the scales. Morpholinium toluene-4-sulphonate shows promise for improving how perovskite solar cells handle electrical charge—no small thing as the world chases cleaner energy.
Any talk about chemicals means thinking beyond the lab bench. Factories producing morpholinium toluene-4-sulphonate must follow careful handling. Skin irritation and respiratory problems can happen with careless exposure, and proper personal protective equipment helps avoid incidents. Disposal becomes another layer. Sulphonates linger in the environment, mixing with water supplies or affecting aquatic life. I’ve toured research sites working on greener synthesis methods and stricter waste management. The technology exists, but compliance and investment lag behind in less regulated markets.
Many chemical suppliers push for batch tracking and transparent safety data sheets, not just to tick boxes, but because businesses want to avoid recalls or regulatory fines. Clear labeling cuts down on confusion among workers and research teams. Several years back, a shop floor mishap due to hazy documentation led to injuries—and a costly shutdown. Lessons got learned, but every company should make safety information easy to find and understand.
Green chemistry has sparked real progress in specialty chemicals. Academic teams test new solvents based on natural sources, aiming to cut pollution without sacrificing performance. Laws around industrial waste and chemical exposure now drive innovation at both the research and manufacturer levels. It’s possible to find similar compounds that break down more easily after use. Some companies run pilot projects to switch formulations, then track performance before scaling up. Results don’t always meet high expectations, but slow gains beat high costs from environmental damage or regulatory penalties.
People often forget that safer choices depend on knowledge—of risks, of substitutes, and of industry best practices. The story of morpholinium toluene-4-sulphonate speaks to the balance between progress and responsibility. Better handling, stronger policy, and ongoing research remain the surest way to make sure usefulness never outweighs safety or environmental care.
Morpholinium Toluene-4-Sulphonate brings together two distinct chemistry worlds: the organic ring of morpholine and the aromatic, sulfonated character of toluene-4-sulfonic acid. Talking structure, the morpholinium part stems from morpholine, a six-membered ring boasting four carbon atoms, an oxygen, and a nitrogen. That nitrogen grabs a proton, forming what’s known as morpholinium. On the other hand, toluene-4-sulfonic acid, the common tosylate used in labs, features a toluene ring (methylbenzene) with a sulfonic acid group hanging off the para position.
The story gets interesting when these two meet. Their ionic bond forms because the sulfonic acid gives up a proton and the morpholine grabs it. The result: morpholinium cation and toluene-4-sulphonate anion linked by ionic attraction. Their chemical formula looks like this: C4H10NO+·C7H7SO3-. In molecular shorthand, you’ll see C11H17NO4S, which reflects everything in the recipe.
Experience in the lab shows that ionic salts like these perform far beyond their molecular curiosity. With morpholinium toluene-4-sulphonate, it’s the blend of hydrophilic (water-loving) and aromatic features that opens up possibilities. The morpholinium cation has a strong attraction for water, enhancing solubility in polar solvents. The toluene-4-sulphonate anion offers stability and a way to anchor the salt in a wider range of chemical techniques.
This kind of structure isn’t just about stability or shelf-life. In my chemical research, choices about salt forms dictate everything from the outcome of a reaction to the process of purifying a product. Switch one member of the salt, and solubility can shift in a way that defines whether something works in an industrial setup. The way morpholinium and toluene-4-sulphonate pair makes this compound particularly useful in pharma, where salts based on organic acids and amines facilitate the production of active pharmaceutical ingredients (APIs).
Chemists use morpholinium salts as phase transfer catalysts, intermediates, or for modulating pH in specialty synthesis. There’s nothing trivial about salt selection. Poor choices in formulation might cause a drug to fall out of solution or behave inconsistently. I’ve noticed that with morpholinium toluene-4-sulphonate, these risks can crop up if researchers ignore purity or overlook the hygroscopic (water-attracting) tendencies of morpholinium salts. Moisture absorption can change how this salt weighs in industrial and lab settings, impacting yields and measurements.
Another area to consider: environmental persistence. Toluene sulfonates stick around in water systems longer than simple salts. Their breakdown takes more effort from wastewater plants. Chemists and industry professionals need practical solutions: using sealed storage for hygroscopic compounds, routine analysis of content, and seeking greener alternatives when possible. Upgrading filtration or using clearer labeling of salt content helps control risk, especially if scaling up beyond the lab bench.
Peer-reviewed research highlights the reliability of morpholinium salts in chemical transformations, particularly for their ease of handling and ability to tune solubility. The US National Library of Medicine and patent literature reference morpholinium toluene-4-sulphonate’s use in synthesis pathways for medicinal agents. Regulatory agencies emphasize the importance of chemical purity, and they track residues from sulfonated compounds in the environment. A hands-on approach—testing storage conditions, monitoring purity, and reviewing waste management—wins out over theory alone.
Morpholinium toluene-4-sulphonate shows up in a range of industrial uses, including chemical synthesis, catalysis, and sometimes in specialty applications like battery production. Its appeal stems from its properties, especially its solubility and ability to aid reactions. But as with many chemicals, questions about safety and toxicity are never far behind.
Reliable information on morpholinium toluene-4-sulphonate doesn’t always pop up at the top of a search, but there’s enough data to raise an eyebrow. Companies that supply the chemical publish safety datasheets, and these often highlight irritation risks—especially to skin, eyes, and respiratory tracts. According to various Safety Data Sheets (SDS) from trusted industry names, this compound can irritate if it contacts bare skin or gets inhaled. That matters on the shop floor as much as in the lab.
Long-term effects don’t seem sharply defined for this particular salt, but data on similar compounds in the morpholinium family make me cautious. Chronic exposure to related chemicals has sometimes shown effects on the liver and nervous system in animal studies. Regulatory bodies, including Europe’s ECHA, have flagged the need for careful handling because certain morpholine derivatives have displayed toxic traits in specific contexts. The absence of solid, long-term studies on this exact compound leaves a gap that’s impossible to ignore.
Lab work and production lines bring real people into direct contact with morpholinium toluene-4-sulphonate. Experience shows that skipping basic safety protocols can lead to avoidable problems like rashes, eye reddening, or coughing fits—not life-threatening, but hardly trivial. I’ve seen folks brush off minor irritation only to slow down work hours later because of some persistent itch or burn. Small incidents accumulate, and this builds up to lost productivity and reduced morale.
Waste and spills create a broader problem, too. Waterborne contamination with amine salts like this one can harm aquatic life. Even if acute toxicity to humans sounds low in summary tables, the ripple effect through the environment puts stress on regulators, neighbors near discharge points, and workers cleaning it up.
Regular exposure reviews go a long way. Factories and labs handle risk best by using closed systems, proper ventilation, and quality gloves. Eyewash stations and clear signage make a difference in risk-heavy areas. Workers benefit from regular training and honest conversations about near-misses. Clear labeling—sticking right to OSHA or REACH standards—keeps people alert to what’s on the shelf or in the drum.
Outside the immediate workplace, chemical producers and users should lean into transparent reporting of spills or accidental exposures to local authorities. Communities trust industries that share data and actively explain how they keep people safe. Looking ahead, shifting to less hazardous alternatives, where possible, drives progress without hiding from cost or performance tradeoffs.
Powerful chemicals like morpholinium toluene-4-sulphonate fuel vital industries, but that power comes bundled with responsibility. My experience says open-eyed management, good habits, and solid infrastructure set the difference between “business as usual” and disaster. Skipping corners rarely pays off. It’s up to leaders and rank-and-file staff alike to keep this chemical useful, not hazardous, for everyone involved.
Morpholinium Toluene-4-Sulphonate isn’t the sort of chemical you want to stash just anywhere. Guided by years working in chemical labs, I’ve seen too many cases where a lack of care leads to ruined stock, dangerous reactions, or wasted money. The first rule for this compound: keep it somewhere cool and dry, out of direct sunlight. Moisture and heat don’t mix well with chemicals like this. Humid air encourages clumping, not to mention the risk of unwanted chemical changes. I’ve always found a dedicated chemical storage cabinet with low humidity best, especially because unexpected reactions can end up burning a hole through more than just your wallet.
Next, think about containers. Only sealed, chemically resistant vessels do justice here. Polyethylene or glass containers tend to last longest with minimal risk of leaching or corrosion. Labels, including the full name, date, and hazard notes, have always been a non-negotiable in every lab I’ve managed. This isn’t just for the sake of the next shift — a missing label can turn routine handling into a guessing game, and nobody needs that kind of stress.
Safety always comes down to simple habits. Gloves and safety glasses matter — it’s easy to dismiss small risks in the name of efficiency, but even minor spills find ways to turn into bigger problems. Morpholinium Toluene-4-Sulphonate powders and granules have a habit of lingering in the air and on surfaces. I’ve watched colleagues get rashes from careless scooping or stirring. Long sleeves and a proper fume hood keep trouble at bay. Cleaning the workspace right after use, with no shortcuts, stops cross-contamination before it gains a foothold.
Scale matters, too. Transferring large amounts can stir up fine particles. Slow and steady pouring, using a scoop designed for chemicals, takes a few seconds longer — but the difference in air quality speaks for itself. I once saw a hasty transfer cloud up an entire work bench, and the cleanup made everyone late for lunch.
Spills don’t ask for permission. A solid spill kit, easy to reach and fully stocked, changes damage control from chaos to routine. I install spill absorbents, disposal bags, and basic PPE in every work area. Training counts here. Running drills, not just giving lectures, builds muscle memory that actually surfaces under stress. In my time on the floor, teams who practiced together always handled accidents smoother and calmer.
The paper trail never feels urgent until things go sideways. Up-to-date safety data sheets belong with every shipment, and updates should never linger at the bottom of an inbox. Regular checks on expiry dates and integrity of containers helped my teams avoid working with spoiled stock. Training goes beyond a single session; it grows from ongoing reminders, review of near misses, and honest conversations about what’s working or not. Workers who know what to expect, and managers who listen, shape the safest environments.
Storing and handling Morpholinium Toluene-4-Sulphonate boils down to habits built from respect for the material and for the people in the space. Investing care upfront saves headaches, money, and maybe even lives. By treating every shipment, transfer, and cleanup as serious business, any team can uphold both health and quality in the workplace.
Ask anyone who spent time in a polymer lab or an industrial battery facility, and they’ll likely know this compound well. Morpholinium Toluene-4-Sulphonate doesn't show up on retail shelves, but it puts in serious work behind the scenes of technologies depended on every day. If you walk through a plastics plant, a research electrochemistry lab, or a specialty coatings facility, there’s a good chance you’re in the orbit of this salt. I remember touring a coatings manufacturer years ago; their R&D folks relied on similar substances to fine-tune resin performance for electronics. With the digitization of everything, these tweaks carry more weight than ever.
Traditional batteries struggle with side reactions and low thermal stability. Electrolyte salts need to improve to push toward safer and more energy-dense batteries, whether for grid storage or cars. Engineers and chemists have tested Morpholinium Toluene-4-Sulphonate as a promising component for advanced electrolytes. It helps maintain good conductivity and stability, making high-performance lithium batteries more reliable. According to scientific reports published in Electrochimica Acta and Journal of Power Sources, using morpholinium salts brings down the risk of degradation, so devices last longer. That matters for people trying to drive 800 kilometers per charge or power a home through the night on stored wind energy.
Anyone who's mixed a batch of acrylic resin knows that small tweaks in additives flip properties for everything from softness to static control. Morpholinium salts, including Toluene-4-Sulphonate variants, get picked for their role as ionic liquids or catalysts in certain polymerizations. The driving factor is often the way these molecules interact with monomers during polymer growth, letting industry create new films, fibers, or containers with specially tuned properties. It’s not always about bigger volumes, but about giving manufacturers a lever to engineer plastics for jobs that didn’t exist a decade ago.
If you’ve ever worked somewhere sparks from static threaten delicate electronics or powder painting lines, you know antistatic agents are not expendable. Morpholinium-based salts, including Toluene-4-Sulphonate types, find application here. Electronics cleanrooms, packaging factories, and some paint manufacturers count on these compounds to avoid static buildup that fries circuits or attracts dust. Their feature set—chemical stability and ability to disperse charge—sets them apart from conventional antistatic additives. This matters more as industries work with smaller, more precise electronic parts.
Drug makers often depend on specialty salts and ionic compounds to run reactions that must be both fast and clean. Morpholinium salts like Toluene-4-Sulphonate serve as phase-transfer catalysts or solvents, helping deliver higher yields, cleaner extractions, or easier purifications. Real-world chemists—myself included—see these tweaks save weeks in research and money in scale-ups. From antibiotics to experimental therapies, anything that trims steps or boosts selectivity builds value both in development and at the pharmacy counter.
Industrial labs and startups pressed for better sustainability now chase alternatives to older and less environmentally friendly chemicals. Morpholinium Toluene-4-Sulphonate lands on lists exploring safer solvents and electrolytes. Real progress depends on sharing toxicological data, greener manufacturing routes, and fairly priced materials. Stronger partnerships between chemical makers and end users help move the industry toward products that last longer and hurt the planet less.
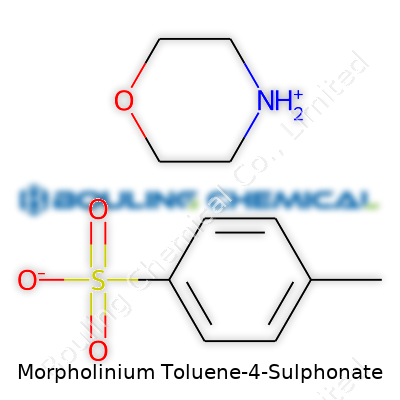
| Names | |
| Preferred IUPAC name | 4-methylbenzenesulfonic acid; morpholine |
| Other names |
4-Toluenesulfonic acid morpholine salt Morpholine p-toluenesulfonate Morpholine tosylate p-Toluenesulfonic acid morpholine salt Morpholinium p-toluenesulfonate |
| Pronunciation | /ˌmɔːrˈfɒlɪniəm təˈluːiːn fɔːlˈsʌlfəˌneɪt/ |
| Identifiers | |
| CAS Number | [3162-58-1] |
| 3D model (JSmol) | `3D model (JSmol)` string for **Morpholinium Toluene-4-Sulphonate**: ``` C1COCCN1.S(=O)(=O)(C6H4CH3)O ``` |
| Beilstein Reference | **1721391** |
| ChEBI | CHEBI:38748 |
| ChEMBL | CHEMBL460099 |
| ChemSpider | 84653 |
| DrugBank | DB11272 |
| ECHA InfoCard | 100.099.600 |
| EC Number | 2527-99-5 |
| Gmelin Reference | 87336 |
| KEGG | C18630 |
| MeSH | C09H19NO3S.C7H8O3S |
| PubChem CID | 137346 |
| RTECS number | WB5950000 |
| UNII | 6Q22T0SO4A |
| UN number | UN2811 |
| Properties | |
| Chemical formula | C4H10NO•C7H8O3S |
| Molar mass | 259.32 g/mol |
| Appearance | White to off-white powder |
| Odor | Odorless |
| Density | 1.24 g/cm3 |
| Solubility in water | Soluble in water |
| log P | -1.02 |
| Acidity (pKa) | 4.8 |
| Basicity (pKb) | 6.0 |
| Magnetic susceptibility (χ) | -75.0e-6 cm³/mol |
| Refractive index (nD) | 1.532 |
| Viscosity | 500 cP |
| Dipole moment | 4.71 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 211.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -820.5 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1615 kJ/mol |
| Pharmacology | |
| ATC code | N02AC51 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P272, P280, P302+P352, P305+P351+P338, P321, P332+P313, P362+P364, P337+P313, P501 |
| NFPA 704 (fire diamond) | 1-2-0-0 |
| Flash point | > 113°C (235°F) |
| Lethal dose or concentration | LD50 oral rat 2000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 1800 mg/kg |
| NIOSH | WU8225000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Morpholinium Toluene-4-Sulphonate: "Not established |
| REL (Recommended) | Not established |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
Morpholine Toluene-4-sulfonic acid Tosylate Morpholinium chloride Morpholinium sulfate |