Morpholine first caught chemists’ attention in the late 1800s, cropping up as a practical byproduct when researchers tinkered with ethylene oxide and ammonia. Nobody really expected much from that sloshy mix at first. Yet by the mid-20th century, industry had started to dig deeper, chasing compounds that could adjust pH or add resilience to rubber. Morpholine’s simple four-carbon, one-oxygen, and one-nitrogen ring seemed to fit the bill. By the 1950s, it found itself steering steam boilers, getting blended into coatings, and even rolling out into crop protection. Over the years, its reputation grew from a lab curiosity to a backbone chemical, sitting in warehouses from Cleveland to Chongqing, and spawning whole lines of derivatives.
Setting a drum of morpholine on the warehouse floor, you’ll notice a thin, clear-to-pale-yellow liquid with a distinctive, ammonia-like scent. One whiff and most chemists remember exactly which room in the lab houses the safety showers. It mixes easily in water, forms a stable base for manufacturing, and works well as both an intermediate and a “fixer” in dozens of processes. Producers typically package it either in heavy-gauge steel drums or bulk tankers, labeling it as a hazardous material, often with UN number 2054. On the shelf, you might see other names: diethylene oximide, tetrahydro-1,4-oxazine, or sometimes simply “morpholine base.” Each bottle refers to the same robust cyclic structure.
Morpholine’s physical properties make it stand out as a utility chemical. It weighs about 87 grams per mole, boils at 129°C, and freezes at a chilly -3°C. It dissolves in water, ethers, and alcohols with ease, which lets it slip into both organic and aqueous reactions. The molecule totes a mildly basic nitrogen atom, meaning it soaks up acids but doesn’t eat through glass or metal flasks the way other bases sometimes do. Its vapor has a sharp, eye-watering kick, so it often gets used in well-ventilated setups or closed systems. With a flash point near 35°C and a strong tendency to crank up corrosion if mishandled, morpholine gets respect in the handling bays of any chemical plant.
Customers look for morpholine in strengths ranging from technical grade to “ultra-pure” for electronics or lab work. Specifications typically demand purity over 99%, a water content under 0.5%, and strict limits on iron, chlorides, and peroxides. Professionals want clear labeling, with hazard statements focused on corrosivity, the need for gloves and goggles, flammability, and environmental risks. Every drum comes with emergency numbers, pictograms for inhalation and contact dangers, and a Material Safety Data Sheet tucked under the cap. In my own work, unmarked containers spell disaster, so most crews double-check the stenciling before opening anything.
These days, most factories produce morpholine by heating diethylene glycol with ammonia over an aluminum oxide catalyst, typically at temperatures approaching 200°C. The process can run batch or continuous, though the latter dominates for industrial-scale needs, since it churns out hundreds of tons with fewer downtime headaches. Some older facilities use a mix of ethanolamine with sulfuric acid, but those setups carry higher costs and disposal issues. The resulting vapor gets distilled and condensed to pull out pure morpholine, with little waste. Facilities monitor temperature, pressure, and catalyst loading closely, since even small slips can mean runaway reactions or clogged pipes.
In the toolbox of organic chemistry, morpholine steps up as both a nucleophile and a building block. It slips into alkylation reactions, making N-substituted morpholines for pharmaceuticals. It works as a precursor for rubber additives, corrosion inhibitors, and dyes. Reacting it with haloalkanes or acid chlorides yields dozens of specialty chemicals. Under oxidizing conditions, morpholine forms N-oxides, which then serve as strong agents in bleaching or synthesis. Researchers lean on it for chiral catalysts, drawing on its ring to steer stereochemistry. Spending time in research, I saw it crop up in everything from pesticide labs to polymer modification benches.
Anyone flipping through catalogs finds morpholine under a slew of names. Some suppliers call it tetrahydro-1,4-oxazine, others list it as 1-oxa-4-azacyclohexane, and older texts note diethylene oximide. Proprietary names sometimes crop up, especially in Europe, but customs paperwork usually recognizes only the basic “morpholine.” This patchwork can trip up newcomers, but anyone familiar with the ring structure gets comfortable with the variations pretty quickly.
Handling morpholine in the field takes vigilance. Skin exposure can bring on burns or rashes in minutes, and vapor inhalation hits respiratory tracts fast. NIOSH and OSHA set occupational exposure limits, and most sites rely on exhaust hoods, splash shields, and chemical-rated gloves as standard gear. Cleanup routines stay strict to contain spills, and companies implement neutralization protocols with vinegar or dilute hydrochloric acid for minor mishaps. Safety drills covering eye washes and decontamination procedures get regular practice. Emergency rooms sometimes see morpholine cases, so clear communication on-site saves lives and keeps agencies happy.
You find morpholine tucked into steam boiler treatments, staving off rust from the inside out. In the coatings industry, it raises pH and stops paint from curdling. Rubber factories prize it for making products last longer in sunlight. Agrochemical makers use morpholine derivatives to stabilize herbicides and fungicides. Printing ink labs grab bottles to keep colors consistent, and even pharmaceutical plants call on morpholine as a backbone for antibiotics or blood pressure drugs. My own experience managing a water treatment line drove home how morpholine set up a reliable chemical “barrier” in closed-circuit cooling systems.
Academic teams keep digging into morpholine chemistry, chasing greener manufacturing processes and looking for new uses in pharmaceuticals and polymers. Recent research examines its potential in lithium-ion battery electrolytes and as a scaffold for new anti-cancer agents. Computer modeling helps predict reactivity for novel transformations. Funding agencies put money behind morpholine fluorination and catalytic upgrading, hoping to cut waste and energy costs. Each tweak in the process blueprint has to pass scrutiny, since the market expects safer, cheaper, and more efficient sources. New patents sprout up every year, exploring everything from crop protection to biocompatible coatings.
Toxicologists track morpholine closely, wary of chronic exposure risks. Lab rats exposed over months show liver and kidney strain, but human studies often point to skin and eye irritation as the main worries in industrial settings. Some derivatives—especially nitrosomorpholine—grab headlines as potential carcinogens, fueling tighter scrutiny on use in food contact materials and agriculture. Regulatory agencies in the EU and US set low limits for direct exposure, requiring industrial sites to monitor air and water releases carefully. I’ve seen operations invest in better glove and mask standards, simple fixes that keep staff safe and comfort compliance officers.
Morpholine sits at a crossroads in global chemicals. On one side, mature applications—boiler treatment, rubber processing, pharmaceuticals—drive steady demand, especially in developing economies upgrading infrastructure and healthcare. Companies eye breakthroughs in battery and agricultural technology, betting that morpholine’s unique mix of water solubility and reactivity opens new doors. Environmental concerns push researchers to craft processes with fewer byproducts and safer handling, shifting toward more sustainable production cycles. With tighter oversight on food and water applications, labs and plants invest in monitoring and containment. In my view, morpholine’s adaptability and long pedigree ensure it isn’t fading from the chemical playbook anytime soon; it just keeps finding new fields to grow in.
Walk past the backrooms of many factories or the ingredient lists of some fruits, and you might stumble across the name morpholine. At first glance, the word sounds like something out of a superhero comic—a strange chemical lurking in shadowy laboratory corners. Yet this colorless liquid plays a role in everyday products far removed from the pages of science fiction.
I spent a summer working at a plant where water quality wasn’t just a nice-to-have, but a question of making sure pipes didn’t eat themselves from the inside out. In that world, morpholine mattered. Factories use it to stop water in boilers from turning acidic and chewing through expensive equipment. Add a dash to the water, and it helps keep metal safe. For anyone handling massive boilers, that simple chemical keeps machines running and breakdowns rare.
Look over in the pharmaceutical world, and morpholine has an entirely different job. Here, it’s more of a builder’s tool. People use it to help piece together complex medications. Sometimes it even forms part of the drug itself. Without these small bits, treatments for infections or diseases stall before they ever reach a hospital shelf.
Head into a grocery store, and morpholine may show up somewhere unexpected. Some countries use morpholine-based coatings on fruits—especially apples—to give them that picture-perfect shine. In my college days, I stocked shelves during midnight shifts and often wondered why some apples looked waxy while others didn’t. That glossy sheen on some imported apples comes from wax mixed with morpholine. The goal? Keep the fruit fresh while it travels thousands of miles. People started asking questions: Was it safe? Health agencies dug into the data, and most found no reason to worry for amounts people actually eat. Canada and the United States allow its use for this purpose, while the European Union keeps it out of their food industry. Food safety often ties into trust, and people want answers close to home when it comes to chemicals on food.
Nobody likes the idea of hidden chemicals. Bring morpholine into a conversation about food or medicine, and folks wonder if there’s a catch. Science says morpholine breaks down quickly in the body and doesn’t stick around to cause harm. Still, the bigger picture comes down to transparent labeling and strong oversight. People want to know what’s in their lunchbox and on their grocery shelves. Companies and regulators ought to work together, keeping the lines of communication open. Labels in plain language boost confidence—no need to bury details in fine print.
Morpholine’s story stands out as an example of chemistry done right when people on all sides—industry experts, health officials, and everyday consumers—talk openly. Build safer processes at the factory, trust science in the supermarket, and treat the public as partners. Knowledge leads to better decisions, whether in a boiler room or an apple orchard. If that’s how chemistry operates, we’re all better for it.
Walk into a facility that uses morpholine and one thing jumps out: this isn’t a casual, all-purpose solvent. People rely on morpholine in making rubber, cleaning agents, and sometimes in food packaging. Despite its range of uses, morpholine can do real damage if someone skips precautions. My early days in a lab taught me how skin burns or breathing trouble aren’t rare accidents—they happen fast and hit hard.
Morpholine has a strong, unpleasant smell. Even a brief encounter can sting your eyes or irritate nasal passages. Direct contact can mean chemical burns and, if it gets in your eyes, it can cause lasting injury. Inhaling its vapors often leads to coughing, dizziness, or headaches. It’s not unheard of for people to forget, get a little careless, and create problems for themselves and coworkers.
Everyone jokes about clunky goggles and tight gloves, but morpholine doesn’t give second chances. Nitrile or neoprene gloves work best since thin latex breaks down fast with this stuff. A splash apron and long sleeves keep nasty surprises off your arms and chest. Safety glasses with side shields do more than people think—they protect against that stray splash or spray, which happens more than managers like to admit. If vapor concentrations go up, using a proper respirator starts to matter. Even the best ventilated rooms can’t always clear strong fumes quickly enough.
Most established sites take air flow seriously. Fume hoods or extraction systems make a difference, and portable fans often push vapors out of work zones. Cracking a window isn’t enough; a local exhaust system keeps the risk down and lets everyone focus on their work instead of their asthma.
Experience shows people skip small steps. They pour morpholine near an open drain or leave containers uncapped. Spills need a neutralizer—often a weak acid solution or a commercial absorbent—never water alone, since a strong reaction can make things worse. Workers should always label every morpholine container, no matter how small, and double-check caps before walking away. Storage should keep morpholine far from acids, oxidizers, or open flames, since mixing it with the wrong stuff means real trouble. Fire is a real risk—morpholine vapors ignite easily, and the liquid burns hotter than people expect.
It’s not enough to hand out safety data sheets and call it a day. Short safety drills and hands-on sessions where people see spills cleaned up or practice a mask fitting go further than printed instructions. People forget rules, especially during a busy shift, or may not know the latest details on how morpholine interacts with new equipment or chemicals. Retraining keeps everyone in the loop and helps keep accidents rare.
Checklists make a difference. Everyone benefits from clear signage, emergency showers, eye-wash stations, and a working plan for spills. Relying on memory alone has never worked well in chemical plants or busy shops. Partnering new folks with seasoned hands brings good habits into routine. Over time, it’s these daily choices and shared experience that prevent painful injuries and costly mistakes.
People in labs and factories often talk about morpholine. Glance at its chemical formula — C4H9NO — and a lot sits beneath those letters. For anyone new, it spells out four carbons, nine hydrogens, a nitrogen atom, and just one oxygen. At the molecular level, it forms a six-membered ring, where four carbon atoms mingle with a nitrogen and an oxygen. This structure doesn’t sprawl like a complicated protein; it wraps itself tightly in a ring, making it pretty unique. The oxygen and nitrogen aren’t just neighbors—they’re directly linked, which lets morpholine behave in ways that a simple amine or ether alone couldn’t match.
The arrangement draws in chemists for a reason. Anyone who has scrubbed glassware knows basicity and solubility turn a headache into a walk in the park. Morpholine’s nitrogen readily accepts a proton, showing off its basic personality. Thanks to the oxygen, it also dissolves in water and a long list of solvents. That’s not something every small compound can brag about. Its cross between an ether and an amine lets it dance in both worlds. This comes in handy every time a reaction calls for gentle conditions or a bit of flexibility.
During my graduate school days, morpholine showed up in the synthesis lab more than once. Its smell sticks with you. I noticed it popped up whenever we faced a tricky substitution or needed a catalyst for corrosion protection. One day, we found our steel equipment pitting from a harsh solvent. The manual suggested morpholine and, like a fix from an old friend, it worked. That’s not just luck—the nitrogen buffers acidic attack and the oxygen makes it rinse out cleanly afterward.
Power plants don’t just rely on engineers. Morpholine plays a quiet but vital role, circulating through boilers and cooling systems to fend off rust. Other anti-corrosion agents struggle in hot, pressurized water, but morpholine outlasts them. The pharmaceutical industry, too, keeps coming back to this ring. Morpholine slips into larger molecules, tweaking their shape or function just enough to turn a dud into a breakthrough. Even pesticides, rubber chemicals, and dyes use it. Its ring acts like a scaffold, allowing chemists to build up bigger compounds or adjust drug properties.
Everything with potential needs checks. Morpholine ends up in wastewater and, without smart treatment, could reach broader waterways. Careful handling — coupled with regular monitoring — can tackle these environmental concerns before they grow. Biodegradation and improved waste capture offer hope, and I’ve watched teams design safer blends so morpholine isn’t left lingering where it shouldn’t be. Labs can push for stricter guidelines, and larger plants have the resources to install better purification tech. The ring that once made jobs easier can still have a future, so long as those using it treat it with the respect any useful—but potent—molecule deserves.
Morpholine shows up in a lot of places, from treating boiler water in industrial plants to helping out with rubber and even medicines. So, you’d think storing it would be old news, but stories keep popping up about warehouses getting things wrong. Mix-ups can lead to leaks, fumes, ruined supplies, and even serious health issues.
The trouble starts with the basics. Morpholine looks pretty harmless at first glance—a colorless liquid with a mild, fishy odor. Then you notice that it’s flammable, reacts with oxidizers, and can cause skin and eye burns. If you catch a whiff, you’ll find out it’s no friend to your respiratory tract either. So anyone stacking drums of morpholine in a dusty corner, or leaving a half-open container next to bleach, is inviting trouble.
I once watched a small manufacturer ignore morpholine safety, storing containers near acids and ammonia, in a cramped shed with no ventilation. Within months, the drums corroded faster than usual. Workers started to notice a sharp, irritating smell, and anything made in the shop took on a strange taint. Not long after, a minor spill led to an expensive clean-up and a few lost paychecks. Local officials got involved.
From what I’ve seen, good storage starts with separation. Morpholine doesn’t mix well with oxidizing agents, acids, or brass and copper. Keeping it in steel or high-density polyethylene containers keeps it stable. Tightly sealed lids cut down on vapor leaks. If there’s one thing to remember from long afternoons scrubbing up chemical spills, it’s that even a tiny puddle of morpholine will travel far and fast if it gets loose.
Temperature control sets the stage for safe chemical storage. Morpholine prefers a cool, dry place, away from direct sunlight. Heat ramps up evaporation, flammable vapors build up, and soon enough, the work environment gets unsafe. I’ve walked into more than one warehouse in the height of summer and found morpholine containers sweating and giving off fumes, just because somebody skipped the climate control.
Airflow also counts. Decent ventilation means a morpholine cloud won’t form if there’s a spill or a slow leak. Simple systems—fans, vents high on the walls, even open windows—help a lot. If the smell sharpens in the storeroom, don’t wait around—find the source, fix the seal, and get it isolated.
Clear labels sound boring, yet they’re the difference between a safe shift and an ER visit. Workers moving quickly reach for cans and drums by habit; easy-to-read labels in plain language prevent swapping morpholine for something harmless. Regular safety training also keeps accidents from becoming the new normal. In a shop where people respect the warning signs and know what to do if something leaks, disasters drop off.
Morpholine storage rarely grabs headlines, but its dangers are real and avoidable. Setting up a separate, ventilated storage space, keeping stocks clearly marked and away from the wrong chemicals, and training every worker who sets foot near it: these steps don’t cost much, but ignoring them can take out a whole week’s profits and punch holes in your safety record. Sometimes, the hidden hazards sitting on the back shelf deserve a second look—even if it means a few hours spent drafting fresh procedures and moving a few barrels.
Morpholine isn’t something most people have heard of, even though it slips into food processing, corrosion protection, and the manufacture of rubber goods. You’ll find it on apple skins, preventing them from shriveling in the store. It shows up in industrial steam lines, fighting off rust. Some producers swear by it for the silky finish it gives waxed fruit. It all sounds innocuous on the surface.
I spent years working in kitchens, reading up on everything that touched the food we served. Discovering morpholine was being used on produce proved unsettling. The World Health Organization issued warnings: high doses hurt lab animals. In certain cases, it reacts with nitrites in food or the environment, forming N-nitrosomorpholine—a compound flagged as carcinogenic. In Europe and Canada, regulators paid attention and slapped down bans or restrictions. In contrast, the United States treats morpholine as “generally recognized as safe” at low levels. Plenty of shoppers wander grocery aisles with little idea their apples might have caught a chemical bath.
People trust that food in the supermarket won’t make them sick. But history shows us that playing fast and loose with chemicals often comes with surprises. The glyphosate debate spiraled for years—safe enough for crops, dangerous, maybe, in other doses. I’m not saying morpholine is glyphosate, but both highlight how science turns up blind spots. Years ago, a parent in my community refused to buy waxed fruit after reading a newspaper feature on food additives. Once you learn what’s on that apple, you don’t forget it.
Industrial waste, runoff, air emissions—morpholine doesn’t just disappear after use. In water, it doesn’t break down quickly. The chemical floats through wastewater systems. Some reports suggest fish and aquatic insects can’t take heavy concentrations. The big fear comes from what’s downstream. If N-nitrosomorpholine forms, contamination stretches further than any local pond. Warnings about environmental impact sometimes get buried behind profit margins. Too often, companies only react after disasters land on the front page.
Morpholine keeps sneaking in because regulators often balance risk with convenience. The rationale: no evidence shows your morning apple is dangerous, so why stir up a fuss? But the evidence on long-term, cumulative exposure still lags behind our use of the chemical. Chronic exposure isn’t well studied. Workers in rubber plants, steam engineers, and farmers deserve better answers. Instead, the pressure to keep produce fresh dominates the conversation. Alternatives exist, from edible coatings made from rice or algae, to improved refrigerated shipping. Some grocery co-ops already insist on unwaxed fruit. These aren’t perfect, but the effort counts.
I’ve learned families want to trust what they eat, and they deserve straight answers. Regulators should push for tighter studies. Lab results should move in step with the stuff showing up in our food and water. Producers could switch to physical preservation methods or natural waxes. Environmental watchdogs need teeth strong enough to hold polluters to account. Public pressure matters. I see how shopper awareness shapes what grocery chains offer. The more real information gets out, the better choices everyone can make. Until then, reading every label and asking hard questions stays essential for anyone serious about health and the environment.
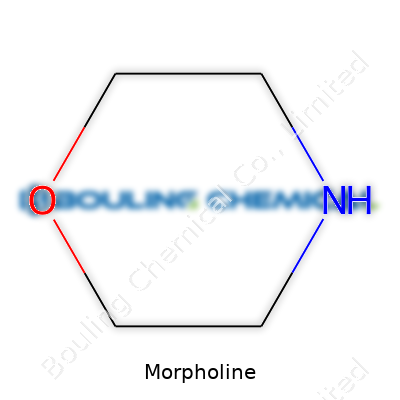
| Names | |
| Preferred IUPAC name | Oxazinane |
| Other names |
Tetrahydro-1,4-oxazine 1,4-Oxaziane Diethylene oximide Tetramethylene imide Morpholin |
| Pronunciation | /ˈmɔːrfəˌliːn/ |
| Identifiers | |
| CAS Number | 110-91-8 |
| 3D model (JSmol) | `CCCCN1CCOCC1` |
| Beilstein Reference | 1209248 |
| ChEBI | CHEBI:27888 |
| ChEMBL | CHEMBL1429 |
| ChemSpider | 3776 |
| DrugBank | DB03255 |
| ECHA InfoCard | 100.006.322 |
| EC Number | 203-815-1 |
| Gmelin Reference | 6227 |
| KEGG | C02340 |
| MeSH | D011019 |
| PubChem CID | 8050 |
| RTECS number | QD0060000 |
| UNII | KB0RSM170W |
| UN number | 2054 |
| Properties | |
| Chemical formula | C4H9NO |
| Molar mass | 87.12 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | Ammonia-like |
| Density | 1.0 g/cm³ |
| Solubility in water | Miscible |
| log P | 0.54 |
| Vapor pressure | 5.3 kPa (at 20 °C) |
| Acidity (pKa) | 8.36 |
| Basicity (pKb) | 7.80 |
| Magnetic susceptibility (χ) | -13.6×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.457 |
| Viscosity | 2.19 mPa·s (at 25 °C) |
| Dipole moment | 1.96 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 222.2 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -335.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1868.7 kJ/mol |
| Pharmacology | |
| ATC code | N04BC04 |
| Hazards | |
| GHS labelling | GHS02, GHS05, GHS06, GHS08 |
| Pictograms | GHS05,GHS06 |
| Signal word | Danger |
| Hazard statements | Hazard statements: "H226, H302, H312, H314 |
| Precautionary statements | P280, P261, P264, P271, P301+P310, P305+P351+P338, P304+P340, P311, P303+P361+P353, P321, P330, P337+P313, P405, P403+P233, P501 |
| NFPA 704 (fire diamond) | 3-2-0 |
| Flash point | 94°C |
| Autoignition temperature | 298 °C (568 °F; 571 K) |
| Explosive limits | 1.8% - 15.2% |
| Lethal dose or concentration | LD50 (oral, rat): 1,050 mg/kg |
| LD50 (median dose) | LD50 (median dose): 1,450 mg/kg (oral, rat) |
| NIOSH | NIOSH: QZ0450000 |
| PEL (Permissible) | PEL = 20 ppm |
| REL (Recommended) | 50 ppm |
| IDLH (Immediate danger) | 140 ppm |
| Related compounds | |
| Related compounds |
Piperidine Thiomorpholine Dioxane Pyrrolidine |