Back in the early 20th century, organic chemists became interested in pyrazine derivatives, exploring their potential in both industrial and pharmaceutical labs. Methyl 3-aminopyrazinecarboxylate didn’t spark immediate headlines, but researchers found it promising as they pushed deeper into heterocyclic chemistry. The carboxylate’s role started small—mostly as an intermediate for agrochemical and pharmaceutical synthesis. Its significance grew as scientists mapped subtle tweaks on the pyrazine ring, eyeing the structure for its nitrogen-rich, electron-donating features. As chemists traded notes and journals filled with reaction schemes, the compound started showing up in more patents, signaling that industries recognizing its potential were scaling up production. In my own time paging through old laboratory notebooks, I’ve seen the transition from a rarely cited curiosity to an established scaffold used in research and development teams worldwide.
The market sees methyl 3-aminopyrazinecarboxylate as a versatile building block. Manufacturers offer it in varying degrees of purity for use in chemical synthesis, medicinal chemistry, and material science. The compound comes as a fine, pale solid, making transport and storage straightforward. Scientists value its reactivity, especially for reactions leading to more complex pharmacophores. In research settings, accessibility often drives discovery. When I first pulled a sample for a thesis project, availability from trusted suppliers made planning easier, removing some of the friction that comes from chasing rare reagents or devising complicated syntheses.
This molecule carries a distinctive aroma, reminiscent of many pyrazine derivatives, with an off-white to light yellow appearance. The chemical structure offers a balance between hydrophilic and hydrophobic properties. A methyl group attached to the carboxylate keeps things compact, while the amino functionality opens doors for further derivatization. Solubility in polar solvents makes it friendlier for reaction work-ups, something researchers value when time matters. Melting point falls within a manageable range for handling, usually just below 200°C. On the bench, its storage remains stable as long as containers stay dry and out of direct sunlight, making it easy to work with compared to more finicky analogs which can degrade or oxidize quickly.
Reputable suppliers post thorough data sheets for methyl 3-aminopyrazinecarboxylate, including spectral characterization—proton and carbon NMR, IR, and MS confirm structure and ensure purity. Labels give clear CAS numbers, batch, and lot references, as well as recommended storage conditions. During one of my projects, a mislabel from a less diligent vendor forced a weeklong setback, highlighting the value of rigorous tracking and labeling in every shipment. Regulatory agencies keep an eye on hazardous labeling, so all containers feature approved pictograms and handling warnings, simplifying risk assessments and inventory management for research and industrial settings.
Lab synthesis typically starts from pyrazinecarboxylic acid or a methyl ester derivative, using selective nitration, reduction, and methylation steps. In commercial operations, optimizing yields involves monitoring pH, reaction temperature, and isolation protocols. I’ve seen lab teams tweak reduction conditions—sometimes going with catalytic hydrogenation, other times leaning into chemical reducing agents—depending on what impurities or byproducts had to be chased out. Equipment setups range from simple batch glassware to multi-liter jacketed vessels for larger-scale manufacturing. After work-up, product purification can call for crystallization or column chromatography, sometimes both. Each stage gets logged—one misstep, and the purity for downstream applications drops.
This carboxylate acts as a flexible hub for synthesis. The amino group stands ready for acylation or alkylation, letting chemists push into new chemical spaces. Cross-coupling reactions benefit from resonance in the pyrazine ring, while the methyl carboxylate can transform into amides or acids as needed in multi-step syntheses. With one batch, I tried nucleophilic aromatic substitution on the ring and got decent yields, but ran into solubility issues until switching to a different solvent system. Modifications enable the creation of analogs for drug screening or agrochemical testing, and each tweak tells chemists more about structure-activity relationships. Experienced researchers quickly learn when to exploit the functional handles or push for more exotic reactivity.
Chemical catalogs add to the name game—methyl 3-aminopyrazine-2-carboxylate, methyl 3-amino-pyrazinecarboxylate, or even by alphabet-soup codes like MAPC. These variations can cause confusion in procurement or literature searches, so accurate cross-referencing matters. During literature reviews, I’ve sometimes run down dead ends searching for just one variant, only to later realize the paper hid the compound under a slightly tweaked name. Suppliers may assign in-house catalog codes, and researchers need to cross-check these against CAS numbers to avoid time lost to mix-ups. In business discussions or patent filings, knowing the alternate names avoids costly mistakes.
While handling this compound doesn’t bring the hazards of strong oxidizers or explosive precursors, safety culture still matters. Proper PPE—gloves, goggles, and lab coats—should be a given. Dust generation can irritate the respiratory tract, and the compound shows mild toxicity on skin contact. In some research environments, the push for larger throughput led teams to reassess ventilation and dust control. Allergic reactions are rare but documented, so personnel training stands front and center to keep everyone healthy. Disposal can’t just go down the drain—follow local chemical waste procedures, log the volumes, and segregate from incompatible waste lines. Safety datasheets outline compatible extinguishing agents and recommended spill control procedures, which come into sharper focus when things go off-script during scaling.
Pharmaceutical research counts methyl 3-aminopyrazinecarboxylate as a reliable starting point for pyrazine-based drugs. Its role in synthesizing anti-tumor and antimicrobial agents comes up often in patent filings. Agrochemical labs use it when developing new fungicides and insecticides. Material scientists have explored it as a functionalized ligand for metal-organic frameworks—early proof-of-concept work suggests further application in catalysis or sensor design. In academic circles, it’s an effective tool for undergraduate and graduate education, since students run basic functional group transformations and quickly get a sense of practical organic synthesis. From a career in both R&D and teaching, I’ve watched this compound unlock both high-throughput screening in big pharma and curiosity-driven projects in small college labs.
R&D teams drive deeper into derivatization to uncover novel therapeutic agents. The pyrazine core’s planarity and electron profile help when docking into biological targets, which IT and computational chemists model before synthesis. I’ve sat through calls where teams debated whether to chase a half-percent yield bump or invest in an entirely new analog. Structure–activity relationship work relies on this backbone, supporting medicinal chemists’ exploration of receptor binding, bioavailability, and metabolic stability. Collaborative programs between academia and industry keep the focus on reducing synthesis steps, cutting reagent costs, and moving to greener solvents. Those changes drive up value, not just profit—training the next generation of scientists in sustainable approaches benefits public and private sectors alike.
Animal and cellular studies suggest moderate toxicity, mostly due to the aromatic amine group. Chronic exposure data lags, raising questions about environmental impact if waste isn’t managed. Published reports run through LD50 data and acute exposure symptoms—respiratory and skin irritation top the list, but so far, long-term carcinogenicity data doesn’t set off alarms. Risk assessments treat the compound with caution, with exposure limits dictated by regional guidelines. During a multi-year trial on analog toxicity, researchers identified metabolic breakdown pathways, including nitrosation products under harsh conditions. Each piece of toxicological data guides SOPs for handling, storage, and waste, keeping scientists and downstream users alert for new data.
Further demand looks likely as scientists search for more targeted pyrazine-based therapies, pushing for molecules with increased efficacy and reduced side effects. Advances in machine learning speed up analog discovery, pointing research teams towards new derivatives of methyl 3-aminopyrazinecarboxylate with untapped application against drug-resistant pathogens or emerging crop pests. Methods for greener synthesis—eliminating toxic solvents and reusing catalysts—shift production to lower environmental footprints. From my experience at industry conferences, collaboration between chemical suppliers, biotechnology startups, and regulatory agencies increasingly drives innovation. Watching this compound move from a specialty reagent to a foundation for next-gen discovery gives me confidence in its continued relevance. Open databases, improved safety data, and a culture of sharing lab notes ensure knowledge flows for years to come, keeping methyl 3-aminopyrazinecarboxylate front and center for innovation in science and manufacturing.
Methyl 3-aminopyrazinecarboxylate rolls off the tongue like a chemistry exam, but in the lab, this molecule represents hope for progress. It sits in the category of heterocyclic compounds—a foundation for modern drug design. Scientists aren’t picking random compounds from the shelf; there’s a reason behind every glass vial. This one links to active research in pharmaceuticals, especially as a starting block for drugs designed to treat infections, inflammation, and sometimes cancer. Seeing a name like this on a research label means the groundwork is being set for therapies that don’t exist yet.
Most people outside research circles never hear about methyl 3-aminopyrazinecarboxylate unless someone’s talking shop at a conference. It matters for two big reasons. The first is its role as an “intermediate”—meaning it helps chemists build more complex compounds. Through simple steps, the lab team can attach new atoms or functional groups to tweak the compound’s properties. This isn’t just academic. Sometimes changing a single nitrogen in a structure can turn a molecule from harmless into a potential cancer-fighter or antibiotic.
The second reason is its ability to make medicinal chemistry more flexible. The world saw antibiotics rocket science forward, but bacteria, viruses, and even cancer keep evolving. Researchers keep testing different arrangements of atoms, searching for shapes that stick to targets in the body. Here, methyl 3-aminopyrazinecarboxylate acts almost like a Lego piece, ready to snap onto other parts the chemists engineer. That flexibility gives researchers real options, not just theoretical ones.
It’s easy to think that science follows a tidy path from discovery to drugstore, but anyone who has worked in pharmaceutical chemistry knows better. Sourcing pure methyl 3-aminopyrazinecarboxylate presents challenges—some suppliers offer high quality, but contamination can derail an experiment. For academic labs with tight budgets, cost poses an extra barrier.
Then comes the scale-up. What works in grams for research rarely translates perfectly to kilograms for manufacturing. Toxic byproducts, waste streams, environmental safety—these come into play each time researchers move to industrial production. The rules for safe handling aren’t just guidelines; they’re lifelines. Pyrazine compounds often bring strong odors and the risk of sensitization or allergic reactions. Safe labs rely on solvent recovery, proper ventilation, and regular safety checks, and those steps sometimes slow down fast-tracking new drug candidates.
Many scientists I’ve talked to see great promise in focusing on molecules like methyl 3-aminopyrazinecarboxylate. It’s not flashy, but it helps push ideas across the finish line. Drug hunters spend years probing molecular shapes, looking for the next clue to better treatments. Here, every new combination could mean a less toxic drug or avoids an antibiotic resistance pitfall.
There’s a lot more collaboration now than there was even a decade ago. Open-source chemistry projects share routes for synthesizing complicated molecules, making research more honest and efficient. Initiatives in green chemistry also seek ways to make these intermediates using fewer solvents and greener reagents. For research and patients alike, that shift matters.
Improving transparency in the chemical supply chain helps trace the source and quality of intermediates, cutting down costly errors. Labs focusing on greener reactions lead the charge by sharing protocols that use less energy and create fewer hazardous byproducts. Connecting researchers through consortia or digital platforms helps swap knowledge about promising modifications. That collective hive mind keeps discovery on track, inching closer to drugs that heal without as much harm.
From my experience, no path to a breakthrough runs smooth, but every step with molecules like methyl 3-aminopyrazinecarboxylate brings tomorrow’s medicine closer to reality.
Methyl 3-Aminopyrazinecarboxylate, often found on a chemist’s shelf, turns up in labs that focus on research and pharmaceutical development. Its chemical formula shows up as C6H7N3O2. That tells us it’s made of six carbons, seven hydrogens, three nitrogens, and two oxygens packed into the same molecule. You add up those atomic weights, and you get a molecular weight of about 153.14 g/mol.
On a dry worksheet, that might just look like numbers. In the actual world, precise knowledge about formulas and molecular weights decides much more — like the way you measure reagents down to the milligram, or how you scale up a process for a drug trial. One slip and the experiment fails, or a purification process doesn’t work out as expected.
In my time dealing with specialty chemicals, learning each molecule’s nature sets the tone for success or disaster. Methyl 3-Aminopyrazinecarboxylate catches the attention of researchers thanks to the building blocks it offers for more complex molecules. Having that amino group and ester group both on a pyrazine core gives chemists plenty of room to play with reactivity and tune potential activity in pharmaceuticals. People working in medicinal chemistry see it as a handy scaffold for making new compounds, and it sometimes gets used to produce antimicrobial agents or experimental drugs. That’s not just speculation; several published studies point to pyrazine derivatives, especially those with amine and carboxyl groups, as promising for anti-tumor and antibacterial paths.
Reliable information on basic specifications like molecular weight means you can trust your dosing calculations during biological testing. Anyone who spends their days wrestling with microgram-level measurements learns the cost of uncertainty very quickly — a decimal misread can waste weeks of work or throw off an entire research direction.
Trusting the numbers from a supplier or database means relying on standardized methods for identification. Analytical chemists use NMR, mass spectrometry, and IR spectra to confirm exact structure and purity. Even minor errors in documentation introduce headaches for everyone along the chain. Lack of clear labeling came up regularly in my earlier days running quality checks, and the fallout meant delays and extra costs for repeat analyses.
Transparency also earns respect — not just from regulators, but from other scientists sharing the same information. Once I learned the value of corroborating published weights or formulas with independent calculations, the number of lab mishaps dropped noticeably. In teaching students or new researchers, I emphasize not to take a supplier’s word at face value — cross-check numbers and watch out for old or conflicting data. That habit builds the competence and confidence expected in science settings that follow rigorous standards.
Trustworthy resources show Methyl 3-Aminopyrazinecarboxylate as C6H7N3O2, clocking in at 153.14 g/mol. Those facts might look basic, but in research and manufacturing, details like this underpin quality, safety, and compliance. Recognizing the foundational facts behind every experiment changes outcomes, from a graduate student’s first synthesis to large-scale drug development projects. Data quality shapes real research and ensures anything built on top stands on solid ground.
My early days in the lab taught me one thing fast—take shortcuts with storage and trouble shows up, fast. With compounds like Methyl 3-Aminopyrazinecarboxylate, you don’t get second chances. If you treat it like a can of soup, purity can drop, contamination sneaks in, and you start guessing whether your whole batch is compromised. Safety matters, and so does getting the most out of every gram.
Room temperature rules work for many chemicals, but ignoring specifics can shave years off the shelf life. Keep Methyl 3-Aminopyrazinecarboxylate at 2 to 8°C for best results. Residential refrigerators can handle this—if you dedicate a shelf and label clearly. Fluctuating temp swings above 25°C can spark unwanted degradation. High summer heat in storage rooms brings hidden risks unless you double-check the thermostat.
This compound attracts moisture. Seal tight. Use airtight containers, preferably those made with glass or high-density polyethylene. Screw-top vials beat snap-caps every time in my experience because a lazy cap lets in enough humidity to cause caking, clumping, or worse—hydrolysis. Adding a fresh silica gel pack doesn’t hurt, especially if your storage area deals with high humidity. Always drive out as much air as possible from containers before closing them up.
Overhead lights in labs can do more harm than good over time. Direct sunlight is almost never a good idea for chemical storage. Use amber glassware or metal containers for Methyl 3-Aminopyrazinecarboxylate, blocking out UV and visible light that can speed up breakdown. I’ve seen more than a few samples turn color or lose their punch after a careless afternoon under harsh lights. Stash the container in a drawer or chemical cabinet when not in use.
Labeling falls by the wayside in a busy setting, but it’s a direct line to safety. Every vial or bottle should carry the name, concentration, received date, and expiry. I learned the hard way after a week-old, unlabeled vial turned out to be something entirely different than what I assumed. Store Methyl 3-Aminopyrazinecarboxylate away from acids, oxidizers, and strong bases. Cross-contamination can be invisible until a run ends up wasted or the fume hood fills with something nasty. Digital and handwritten logs help track who opened what and when.
Even with tidy storage, spills can happen. Keep spill kits near storage zones, stocked with material suitable for absorbing organic chemicals. Good ventilation, chemical-resistant gloves, and goggles deserve a home next to your storage shelf, not locked away in a distant supply closet. A clear, practiced plan beats searching frantically for a mop in a moment of panic.
Dependable storage combines vigilance with the right materials. Temperatures between 2 to 8°C, airtight and light-resistant containers, solid labeling, and separation from incompatible substances all cut down on loss and risk. Each time a sample survives months on the shelf and still performs, it tells me careful storage pays off in ways documentation alone can’t measure.
Scientists and manufacturers don’t just grab any chemical off the shelf. Purity shapes how research, formulations, and final products turn out. With Methyl 3-Aminopyrazinecarboxylate, labs and companies pay serious attention to what grade they’re using because the smallest impurity can throw off a reaction or wreck the consistency of a pharmaceutical batch. My time spent in a research lab taught me that even tiny contaminants can sabotage an entire week’s experiments.
It isn’t just about a single “lab grade” versus “technical grade.” Grades exist along a spectrum: you’ll find pharmaceutical grade, analytical grade, and sometimes custom purities tailored by suppliers. Analytical grade provides that near-spotless composition scientists count on, often hitting 99% or above. Pharmaceutical applications step even higher, matching strict regulations to rule out any surprises at the testing stage.
Industrial settings don’t always need that level of perfection. If you’re working on an agricultural compound, pigments, or a pilot run, technical grade might do the trick. These have minor impurities that, in those contexts, won’t affect results. Chemists from several industries share this pattern: select the lowest usable grade to reduce costs without compromising quality.
The conversation around purity isn’t just academic. Real-world stakes get high in healthcare or food. I remember one case: a minor contaminant snuck into a batch of an intermediate chemical. This delay mushroomed into weeks of extra testing and regulatory headaches, not to mention sinking trust from end users. Cleaner material cuts down that risk. The FDA’s guidelines on active pharmaceutical ingredients make it clear—producers need tight control over starting materials, covering both visible impurities and those hard-to-spot trace elements.
Not every supplier meets the same benchmarks. Certificates of analysis (COA) and third-party lab verification help buyers avoid surprises. A COA breaks down the content of each batch, laying out detectable impurities, moisture content, and other properties. It’s a safety net, especially for regulated sectors. My own habit: don’t accept a shipment unless the COA matches both your order and your own test results.
The price tag on high-purity Methyl 3-Aminopyrazinecarboxylate can put off some buyers, especially early-stage startups or resource-strapped research groups. Yet, that extra spend pays back in reliability. Failed syntheses, batch recalls, or regulatory penalties often cost much more than upgrading to purer raw materials. In my years talking shop with procurement teams, no one ever wished they’d chosen the cheapest grade once trouble struck.
Increasing transparency across chemical supply chains, investing in trustworthy sources, and building partnerships with reputable suppliers all contribute to safer labs and stronger products. The importance of honesty about material quality keeps growing, especially as regulations become tighter. Companies willing to spend a bit more for verified high-purity chemicals, like Methyl 3-Aminopyrazinecarboxylate, make fewer mistakes down the line and outpace competitors stuck fixing preventable issues.
Methyl 3-Aminopyrazinecarboxylate doesn’t show up on most people’s radars, yet for folks in chemistry labs or industries using this compound, its safety profile deserves real attention. You deal with white to off-white powders like this one, and the risk doesn’t shout at you like a jug of concentrated sulfuric acid, but hidden hazards never play fair with complacency.
This compound is an organic intermediate that takes part in pharmaceutical synthesis and research projects. It brings a subtle but real threat: inhaling dust, accidental skin contact, or splashing solutions during transfer and weighing comes with health dangers. Several routes to harm show up if you forget about personal protection or skip safety steps, and labeling this as just another “lab chemical” risks overlooking the particulars that make handling it tricky.
Experience has shown me that routine builds comfort fast, and comfort brings risk. Gloves and goggles are mandatory – not “sometimes” gear. Even short exposure can cause skin irritation, and dust in the eyes burns more than you expect. If you work in cramped, older labs or spaces without strong airflow, breathing in fine powders gets easy. Use local exhaust ventilation or at least keep to a fume hood, especially during weighing or mixing. Eyes and lungs always thank you for good habits; the sting of a careless dust cloud isn’t worth seconds saved.
Don’t grab any gloves off the rack. Nitrile offers solid protection against organics, while standard latex gloves can let smaller molecules slip by and cause skin irritation over a long shift. Aprons and long sleeves cut the risk of accidental drips and splashes on your clothes, since even a “mild” irritant soaks in fast.
Incidents don’t just happen on the big scale. A cracked balance pan, a fumbled scoop, a leaking vial cap – small mistakes lead to spills that turn into contamination issues. One slip, and you’re cleaning powder out of drawer seams for weeks. Chemical exposure isn’t the only issue: cross-contamination with other sensitive research can ruin a day’s experiments or worse, skew results nobody catches in time. Label all containers clearly; the world doesn’t need another “unknown white powder” scare that throws off a whole work group.
I’ve seen old-style chemical storage rooms, sometimes more cluttered than a college dorm. Storing compounds like Methyl 3-Aminopyrazinecarboxylate away from strong acids, oxidizers, and moisture matters. Dry, cool cabinets with warning labels help minimize risks. Unmarked bags or mystery bottles stashed behind other supplies don’t cut it, no matter how crowded the shelf gets.
Spilled powder goes beyond a quick swipe with a paper towel. Sweep up gently, avoid raising dust, and use a wet cloth for final cleanup. All contaminated materials – wipes, gloves, even lab coats that faced spills – need disposal in labeled hazardous waste bags. Regular trash cans send lab waste straight to where nobody wants it. Training new students or staff always includes the point about waste: cutting corners with disposal courts disaster down the line.
It takes more than equipment and rules to keep people safe. A good lab or plant culture means watching out for colleagues, reminding each other to keep gloves up and goggles on, and taking the extra minute to double-check storage and labels. Strong habits make a difference. Handling chemicals like Methyl 3-Aminopyrazinecarboxylate with respect supports not just your health, but everybody’s research, workplace, and even reputation. Everyone deserves to finish the day healthy, with the lab running smooth and orderly for tomorrow.
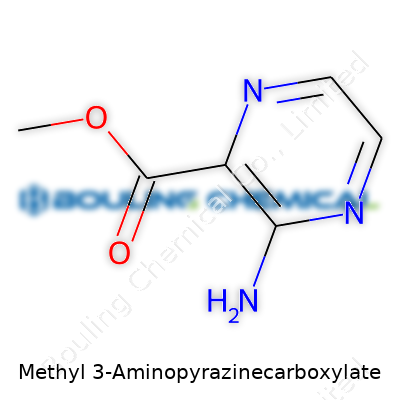
| Names | |
| Preferred IUPAC name | Methyl 3-aminopyrazine-2-carboxylate |
| Other names |
NSC 73650 3-Aminopyrazinecarboxylic acid methyl ester Methyl 3-aminopyrazine-2-carboxylate |
| Pronunciation | /ˈmɛθɪl θri æˈmiːnoʊ paɪˈræziːn kɑːrˈbɒksɪˌleɪt/ |
| Identifiers | |
| CAS Number | 38895-04-0 |
| 3D model (JSmol) | `3D model (JSmol)` string for **Methyl 3-Aminopyrazinecarboxylate**: ``` CN1C=NC=C1C(=O)OC ``` (Note: This is the SMILES string, commonly used to represent 3D chemical structures for molecular visualization tools like JSmol.) |
| Beilstein Reference | 107211 |
| ChEBI | CHEBI:91215 |
| ChEMBL | CHEMBL243672 |
| ChemSpider | 16216572 |
| DrugBank | DB08184 |
| ECHA InfoCard | 15e827ca-3c2a-4851-9f6e-aca52ddfb617 |
| EC Number | 87603-98-7 |
| Gmelin Reference | 110838 |
| KEGG | C14337 |
| MeSH | D017019 |
| PubChem CID | 15242676 |
| RTECS number | UY7521000 |
| UNII | 8W5B3IP8C2 |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | `DTXSID20885552` |
| Properties | |
| Chemical formula | C6H7N3O2 |
| Molar mass | 151.14 g/mol |
| Appearance | Light yellow solid |
| Odor | Odorless |
| Density | 1.34 g/cm3 |
| Solubility in water | Slightly soluble |
| log P | -0.28 |
| Acidity (pKa) | pKa = 3.08 |
| Basicity (pKb) | “pKb = 11.6” |
| Magnetic susceptibility (χ) | -47.7e-6 cm³/mol |
| Refractive index (nD) | 1.560 |
| Dipole moment | 3.79 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 309.6 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | N06AX19 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes serious eye irritation, may cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302 + H315 + H319 + H335 |
| Precautionary statements | P261, P264, P271, P272, P280, P302+P352, P321, P362+P364, P501 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 135.6°C |
| NIOSH | RN9840000 |
| PEL (Permissible) | Not Established |
| REL (Recommended) | 10 mg |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
Pyrazinecarboxylic acid 3-Aminopyrazine-2-carboxylic acid Ethyl 3-aminopyrazinecarboxylate Methyl 2-aminopyrazinecarboxylate 3-Aminopyrazine Methyl pyrazinecarboxylate 3-Aminopyridine |