Looking back over decades, the pursuit of new organic compounds kicked off all sorts of breakthroughs in chemistry and pharmaceuticals. Methyl 3-amino-4-methyl-2-thenoate popped up as part of a growing curiosity about thiophene derivatives. Back in the mid-20th century, chemists noticed that swapping groups around the thienyl ring yielded unexpected reactivity. By the late 1970s, publications began recording synthesis routes for thienoic methyl esters. Progress snowballed as research teams in Europe and Asia started exploring these compounds for their building block value. Developing efficient ways to introduce amino and methyl groups onto thiophene esters presented serious challenges. Yet demand for advanced intermediates pushed industry to refine their synthetic processes. Before long, this small but essential molecule earned a place in the catalogs of both academic labs and specialty fine chemical suppliers.
Methyl 3-amino-4-methyl-2-thenoate counts as a thiophene derivative featuring both an ester and an amino group. With its combination of functional groups, this compound offers a foundation for a wide range of downstream applications. In daily research, folks return to this molecule anytime they want to build more complex thiophene-based structures. The chemical’s importance often comes down to the versatility of the thiophene ring system and the way its substituents influence electronic and steric properties. Even among researchers set on radically different outcomes—antibiotics, flame retardants, dyes—this building block finds new utility. In my own experience, introducing the methyl group tends to change reactivity, which can be the difference between a dead-end synthesis and a breakthrough.
In a solid state, Methyl 3-amino-4-methyl-2-thenoate appears as a pale-yellow crystalline powder, distinct enough to be identifiable without complex instrumentation. It offers a melting point usually in the range of 70–75°C, although this varies slightly with purity. This compound dissolves readily in common organic solvents such as methanol, dichloromethane, and ethyl acetate. Water solubility stays modest, which can slow down certain purification techniques, but column chromatography can manage the task. Chemical structure analysis reveals the thiophene ring confers a bit of aromatic stability, while both the amino and ester groups allow for a variety of substitution and modification strategies, critical when customizing molecules for different scientific needs. From a chemical reactivity perspective, the amino group acts as a nucleophile, while the ester brings possibilities for hydrolysis or conversion to other carboxyl derivatives.
Quality standards for this compound follow both internal controls and national regulations. Common purity levels exceed 98%, and advanced chromatography routinely verifies this before shipping. On commercial labels, suppliers note the CAS number, molecular weight, empirical formula (C7H9NO2S), recommended storage at 2–8°C, batch number, and expiration date. Important warnings flag the need for gloves and goggles. The storage note comes from the compound’s sensitivity to moisture, which can nudge hydrolysis reactions if left unchecked. Many manufacturers provide certificates of analysis, so research teams can cross-reference their own data. Documentation must confirm that no significant impurities disrupt planned chemical transformations. The details listed on the label aren’t just there for compliance; they make troubleshooting in the lab much less frustrating.
Synthetic routes to Methyl 3-amino-4-methyl-2-thenoate usually start with 4-methylthiophene as the core. Nitration under controlled temperature leads to 3-nitro-4-methylthiophene. Catalytic hydrogenation transforms that nitro group into the essential amino group. This step tends to require a decent catalyst, like palladium on carbon, and hydrogen gas, with careful attention to pressure and flow for consistent yield. The final stage involves esterification, typically with methanol and an activating agent such as thionyl chloride or acid anhydride to produce the methyl ester. Each stage in this pathway needs rigorous purification because leftover starting materials and byproducts can trip up future synthesis steps. Chemists favor these methods for their scalability and the ability to tweak for higher yields, making lab-scale or industrial-scale implementation practical.
Once in hand, the molecule welcomes a host of transformations. The amino group opens doors to acylation and alkylation, which matter in pharmaceutical discovery for generating compound libraries. Esters transform through classic hydrolysis, yielding carboxylic acids that take part in further coupling reactions. The methyl substituent at the four-position blocks ortho reactivity, steering incoming groups towards other open sites. Chemists craft sulfonamide, amide, and urea derivatives with relative ease thanks to the accessible amino moiety. Each modification potentially unlocks new biological activity, so folks in drug discovery often use the molecule as their scaffold. In my work with materials chemistry, functionalizing the ester has let us fine-tune processability in polymer blends, demonstrating just how much versatility comes baked into such a small molecule.
Depending on the catalog or research article, the compound might show up as 3-Amino-4-methyl-2-thiophenecarboxylic acid methyl ester, Methyl 3-amino-4-methylthiophene-2-carboxylate, or just as its systematic IUPAC name. Some suppliers shorten it to MATME. The spread of synonyms often causes confusion, especially for researchers ordering through international suppliers. In laboratory databases, clear cross-referencing between names, CAS numbers, and structural diagrams helps avoid ordering mistakes. For an early-career researcher, getting the wrong compound because of tricky synonym use wastes both funds and time.
Using Methyl 3-amino-4-methyl-2-thenoate calls for level-headed attention to personal protective gear—nitrile gloves, lab coats, and safety goggles. Its handling protocols reflect those of other aromatic amines: minimize skin contact, limit inhalation, and keep storage vessels tightly sealed. Researchers working on gram scales need solid ventilation and ready access to spill kits. Safety sheets warn of moderate toxicity by ingestion and irritation with direct exposure. Disposal follows regional chemical waste policies, often through licensed hazardous waste contractors. For shipping and large-scale use, documentation must travel with packages, giving emergency responders the information they need to act. In day-to-day lab work, little moments like washing glassware or weighing powders can become safety issues if rushed, so training and clear signage help sidestep accidents.
Medicinal chemists turn to this building block for synthesizing anti-infective and anti-inflammatory drug candidates. Functionalized thiophenes harness unique electron distributions, offering routes to enzyme inhibitors and receptors that would otherwise resist conventional ligands. Agrochemical companies explore its utility in herbicide and fungicide discovery programs. In my own experience, the value often shows up at the interface of academia and industry—academic labs test novel analogs for unexpected activity, and industry teams scale up lead compounds for preclinical work. The ester’s reactivity supports both chemical screens and targeted modifications, so it tends to show up in exploratory studies looking for next-generation materials, dyes, or even organic semiconductors.
R&D efforts often focus on process optimizations, such as reducing byproduct formation and improving atom economy. Programmable synthesis platforms, which automate reaction monitoring and purification, help teams targeting larger compound libraries for high-throughput screening. Analytical teams push for more sensitive detection of trace contaminants, knowing even minor impurities can skew biological assays. Research groups interested in green chemistry seek out solvent systems that reduce environmental impact. Cross-disciplinary collaborations pull in computational chemists, who model how substituents at the three and four positions impact binding to target proteins or alter electronic properties. Instead of sticking with classic routes, some innovators have tried bio-catalysis and microwave-assisted synthesis to cut down energy use and cycle times.
Toxicologists test this molecule using standard models, like cell line cytotoxicity and rodent studies, to flag acute and chronic hazards. Early data point to low but notable toxicity; this isn’t a compound for casual handling. The amino group raises red flags for possible mutagenicity or unwanted metabolic activation, so teams regularly run Ames tests and in vivo follow-ups. Advanced mass spectrometry tracks metabolic breakdown to catch any hidden risks from reactive intermediates. Organizations carrying out repeated handling implement health monitoring, and regulatory frameworks call for closed-system handling beyond certain quantities. For most labs, comprehensive records of exposure history and waste management help maintain a safe environment. Personally, treating every unfamiliar compound as potentially hazardous has avoided more than a few occupational health incidents in the lab.
The future for Methyl 3-amino-4-methyl-2-thenoate looks tied to advances in precision medicine, sustainable synthesis, and materials science. Researchers in drug discovery keep developing analogs, hoping to break through current barriers in antibiotic resistance. As green chemistry gains more influence, focus will shift to cleaner, solvent-free synthesis and higher-yield processes. In data-driven chemistry, machine learning platforms will likely predict better derivatives and highlight synthetic shortcuts, boosting both productivity and safety. The compound’s basic framework offers plenty of room for further functionalization, so work in organic electronics and optical materials can develop alongside pharmaceutical efforts. Demand for such versatile intermediates grows as the gaps between lab-scale insight and industrial manufacturing shrink. My own outlook always looks for the ways improved cooperation between chemists and engineers will bring out new applications from familiar molecules like this one.
The name Methyl 3-Amino-4-Methyl-2-Thenoate doesn’t roll off the tongue. Still, anyone who’s poked around a pharmaceutical lab or tested new compounds in a university setting will probably recognize similar names all over reaction logs. This compound slips into synthetic routes for a clear reason: it helps chemists shape new molecules for medicine, agriculture, and even advanced materials.
Molecules like this usually enter the scene as intermediates. In a crowded chemical reaction, intermediates set up specific connections between atoms or offer functional groups—a chemical term for the sticky ends that latch onto other atoms present during the process. Scientists design them to hand off important parts for creating new drugs. Having used related molecules as stepping stones in small-scale synthesis, I’ve seen first-hand how key intermediates make tricky reactions possible. Without them, researchers sometimes hit dead ends, unable to push through bottlenecks that block a route to a useful medicine.
Methyl 3-Amino-4-Methyl-2-Thenoate’s biggest claim to fame traces back to drug discovery. The pharmaceutical world thrives on new molecular patterns. Developers hunt for small tweaks to improve safety, lower side effects, or boost how well a medicine works. The ester and amino parts of this molecule open lots of options for change. In real projects, chemists will bolt new groups onto molecules like this one, seeing how each version affects biological tests. Sometimes these experiments stumble onto new antibiotics or anti-inflammatories. Reports in research journals show that thienoate compounds—sharing the same “thieno” backbone—have popped up in efforts to block enzymes or slow down infections.
In graduate work, I learned how small structural changes can steer the entire path of a project. Several years back, a few peers chased new cancer treatments using related chemicals, building off the same sulfur-containing ring. They didn’t discover a blockbuster, but the method helped other teams move forward. Each effort builds a little more knowledge, which gets recycled in other experiments and sometimes put to use making a finished drug.
Even outside headline pharmaceutical projects, chemistry students and professionals often work with intermediates like Methyl 3-Amino-4-Methyl-2-Thenoate. Some laboratories use this compound as a starting material to explore organic chemistry’s big themes: new reaction tricks, creative routes to rare compounds, or ways to build molecules more safely. Its ring structure gives stability, making it easier to handle. This brings down waste and lets even cash-strapped college labs try out reactions that, not so long ago, only a few top universities could afford to repeat.
Experts know supply chain security always matters. Recent global disruptions showed how missing one intermediate can hold up entire drug launches. Producers who source molecules like these from reliable partners avoid disaster down the line. If supply stalls, researchers can waste months waiting for someone to resupply rare starting materials. Locally made or well-documented intermediates promise greater control. I’ve watched teams scramble after a single shipment got stuck at customs—a reminder the world depends on these basic building blocks.
Safety never leaves the table. Chemicals like Methyl 3-Amino-4-Methyl-2-Thenoate sometimes demand special handling—personal experience tells me that good ventilation and solid goggles aren't optional. Green chemistry looks toward milder reactions and less hazardous waste. Improving environmental practices involves making sturdy intermediates that don’t generate a dozen byproducts that nobody wants. I’ve spoken to chemists who make safer, smarter choices because they care about what the next generation will inherit. Investing in new synthetic methods can cut both costs and risks, which makes life a little better inside and outside the lab.
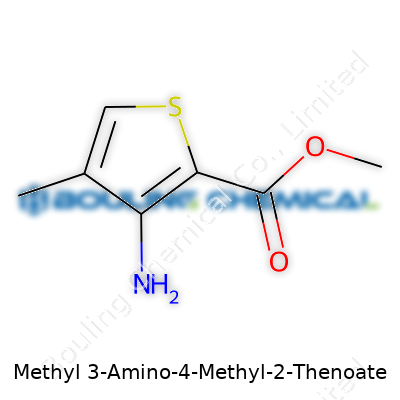
Methyl 3-Amino-4-Methyl-2-Thiophenecarboxylate carries a fairly complex name, so it helps to look at each piece to understand the whole. The backbone comes from thiophene, a five-membered ring that includes four carbon atoms and a single sulfur atom. Chemists tend to appreciate thiophenes because they mimic benzene in stability but bring a bit more twist thanks to that sulfur atom.
On this thiophene ring, modifications appear at three spots. At position two, the molecule holds a carboxylate group in the form of a methyl ester. At position three, you find an amino group. At position four, a methyl group attaches. Altogether, these pieces shape the properties and uses of the compound.
Imagine thiophene’s pentagonal ring: sulfur at the top point. Counting from sulfur as the number one position, the methyl ester group attaches to the second carbon, the amino group to the third, and the methyl group to the fourth. Chemists note each shift in the ring's electronic balance alters how the molecule reacts with others.
The methyl ester group provides chemical stability and changes the solubility profile, which matters a lot in reactions, especially if the compound finds itself used in pharmaceuticals or dyes. The amino group offers a strong site for further chemical connection, letting scientists attach more pieces or tweak the molecule for new applications. The methyl group brings subtle shifts to physical properties. None of these changes stays isolated; together, they flavor how the molecule behaves in real-life chemistry.
A molecule’s structure decides the work it can do. In my own experience as a researcher, even a small change—say, moving that methyl group from the fourth spot to the fifth—can swing a compound’s effectiveness or safety. Chemists often use thiophene-based molecules as stepping stones in creating pharmaceuticals, agrochemicals, or conducting polymers, where electrical conductivity and biological activity intertwine.
Facts from research journals show that methyl esters often help a molecule cross biological membranes, which widens the list of environments where the compound proves useful. An amino group makes the molecule more amenable to coupling with other building blocks. Adding a methyl group improves shelf stability. This combination supports studies aiming to build better antibiotics or to craft materials for electronics that don’t break down quickly in air or light.
Sometimes, the key challenge with such molecules involves purity and synthesis cost. Impurities in commercial batches can throw off experimental results or even trigger unwanted side effects in early drug studies. The answer often comes from refining synthetic methods—using greener solvents, easier purification steps, or catalysts that cut down on energy waste. I’ve watched labs switch to flow chemistry techniques to scale up such thiophene compounds while reducing pollution.
As the world leans more on tailored materials, researchers stand to gain from thinking deeply about each tweak on a molecule’s ring. Methyl 3-Amino-4-Methyl-2-Thiophenecarboxylate gives one small but vivid example of how precise chemical structures open up new fields of invention and discovery. The details in its construction aren’t minor quirks—they’re the launch pad for progress.
Handling chemicals like Methyl 3-Amino-4-Methyl-2-Thenoate doesn’t offer any shortcuts. My years hunched over a fume hood taught me to treat every reagent with respect, especially those with limited toxicity data. Even if the reports seem thin, that doesn't make a compound harmless. A quick splash on bare skin or a whiff of dust often carries consequences people don’t think of until it’s too late. Simple carelessness can turn a regular working day into a visit to the occupational health office.
Before opening a bottle, I always check my personal protective gear. Chemical-resistant gloves come before curiosity. Nitrile usually serves well, but chemicals sometimes seep where latex falls short. Safety goggles help avoid splashes that burn or blind. Without a thick lab coat and closed shoes, I’d be more worried about accidents. In hot, crowded labs, it’s tempting to skip protection, but that’s when injuries happen.
Good airflow keeps you from breathing in dust, fumes, or vapors. Years of managing ventilation issues taught me to never trust a silent fume hood; if I can’t see tissue flutter or hear the draft, I talk to maintenance. Regular checks make a real difference. I see more value in a well-placed carbon filter or HEPA extractor than a fancy analytical instrument when working with volatile or dust-producing solids.
Small spills become big problems on cluttered benches. I grew into the habit of wiping workspaces before and after handling anything reactive. Proper labeling stands as a lifesaver. Unmarked vials can lead to guessing games no one wants to play, especially during late-night syntheses. Even seasoned researchers pick up the wrong container when hurrying or distracted.
Training never felt like wasted time. Explaining to new lab members why we keep calcium gluconate for hydrofluoric acid or why we never use water on alkali metal spills gets the point across. Safety drills, even if repetitive, made sure everyone reacted quickly during a spill.
Disposing of chemical waste crops up every time I mix a batch. Pouring organic solvents down the drain doesn’t just annoy the city, it endangers wildlife and ground water. My standard protocol stores liquid and solid wastes in marked containers; nothing leaves the lab until the hazardous waste crew collects it. Keeping SDS sheets handy gives me peace of mind. When something goes wrong, clarity vanishes, and those documents lay out symptoms and recommended action without delay.
No one ever regrets careful planning. I check my inventory, make sure my workspace is clear, line up all equipment, and keep emergency showers and eyewash stations within reach every single time. Routine builds muscle memory, and muscle memory saves lives.
Safety isn’t about paranoia. It’s about protecting people so their work matters tomorrow, not just today. Respect for every chemical—no matter how small the batch or simple the structure—keeps science moving forward and workers heading home unharmed. That’s a lesson worth repeating in every lab, on every shift.
Methyl 3-Amino-4-Methyl-2-Thenoate, a chemical often found in research labs and some niche manufacturing settings, demands respect. Anyone who’s spent time in a chemistry lab has tangled with similar compounds, from aromatic esters to various amines. Working day in, day out with these materials, I learned one thing early: underestimating storage risks can mess up more than just an experiment. This chemical packs risks—think irritation for skin and eyes, plus volatility that increases when the environment’s too warm or compromised by moisture.
Storing materials like this just anywhere on a shelf invites trouble. Any material prone to degradation, contamination, or volatility benefits from cool, dry storage away from direct sunlight. If humidity seeps into the mix, the ester group can hydrolyze, which jeopardizes purity. In my years of lab work, failures often traced back to overlooking the basics—like failing to check the seals on a container or storing a volatile product near a window. Direct sunlight raises temperatures in unpredictable ways, and just one unexpected reaction can result in hazardous fumes or inactive material.
The walls of a proper storage cabinet echo with the rattle of tightly sealed glass bottles and chemical-resistant polymer flasks—never the flimsy or questionable. Over time, I saw leaks and fumes in labs that cut costs or skipped best practices. That never ends well. This chemical interacts strongly with the wrong kind of plastic. Stick with glass, Teflon-lined lids, or materials specifically rated for amines and organosulfur compounds. Weak seals allow vapor escape or moisture intrusion, both of which can trigger a safety incident.
Too often, folks overlook compatibility charts. I once watched a careless mix-up—mild-mannered at first—turn into a scary exothermic event when amines sat too close to strong acids or oxidizers. Avoiding cross-reactions means storing Methyl 3-Amino-4-Methyl-2-Thenoate away from reactive or corrosive agents and preventing careless spills. Even a dribble of bleach or nitric acid nearby can set off a cascade of problems.
Failure to label containers is a recipe for confusion and—worse—accidentally mixing up substances. Every container in my labs bore clear, honest labels: full chemical name, concentration, storage date, and hazard information. No shortcuts. This practice doesn’t just help the current team; it sets the standard for anyone following or auditing later. Access should also be limited to trained people. A clear logbook and inventory system allow for quick assessment—so if storage conditions need fixing, you know before things escalate.
Safe storage only holds up when everyone in the loop understands what’s at stake. Regular safety training keeps the team up-to-date on procedures, emergency protocols, and changes in chemical guidelines. I’ve seen the fallout from inadequate planning—people only remember the eyewash station’s existence when something goes wrong. Fire extinguishers, fume hoods, and spill kits should stand ready and accessible. Safety documentation needs regular reviews, with updates coming from trusted regulatory guidance like the CDC and OSHA.
Storing chemicals safely never boils down to a checklist. It’s about building a culture where people look out for each other. In every organization where I felt proud to work, storage wasn’t an afterthought. It became a habit, almost like muscle memory, to double-check containers, log changes, and question anything that looked out of place. That culture, backed up by facts and consistent training, puts safety first—always.
Lab work moves fast, but the wrong compound can send a project off the rails. Methyl 3-amino-4-methyl-2-thenoate isn’t some household aspirin ingredient; this molecule—sometimes suggested for pharmaceutical uses or advanced chemical synthesis—calls for precision in its makeup. Sloppy chemistry at the front end makes for unreliable results or wasted time down the line.
The accepted norm pegs chemical purity higher than 98% for compounds in pharma or research. Low purity brings unnecessary risks: imagine building a tower from bricks full of clay rather than sturdy stone. Each impurity might gum up a reaction or skew data. High-end suppliers hand over a certificate of analysis with gas chromatography and HPLC numbers, showing just how much usable compound hits the bottle.
Impurities can lurk, showing up as moisture, residual solvents or leftover starting materials. Water is a big offender here, as moisture can alter reaction rates or, worse, hydrolyze sensitive esters. Suppliers put special emphasis on “loss on drying” (LOD). Large swings in LOD point to poor storage or weak drying steps, which no lab wants when accuracy counts.
Authenticity depends on more than a label. Skilled chemists check melting point ranges—solid forms should land squarely inside a window listed on safety data sheets. Off-the-mark melting points often signal contamination or a rogue polymorph. Appearance counts too. Most labs expect fine white to pale yellow powders, not clumpy, off-color dust. Color shifts warn that air, light or heat might have set off slow decomposition.
Infrared (IR) and nuclear magnetic resonance (NMR) serve as the gold standard for deeper identity checks. Spectra from these methods trace a fingerprint unique to the molecule, flagging strange peaks for unexpected byproducts. Testers also use mass spectrometry, confirming no stray fragments from incomplete syntheses hang around to skew results.
The quiet offenders—trace elements and solvents—often slip through if a supplier tampers with shortcuts. For anything entering biological testing, guidelines mirrored after ICH Q3D call for less than 10 ppm for toxic metals such as lead, arsenic, mercury, and cadmium. Too much metal doesn’t just raise regulatory flags, it can wreck enzyme-based assays and contaminate final products.
Solvent residues challenge quality teams just as much. Producers push for levels beneath the limits given in ICH Q3C (often under 500 ppm for most solvents). Reliable suppliers take care to use easy-to-purge solvents during synthesis, reducing the clean-up burden. Labs now run GC-MS tests as routine, refusing compounds that carry too much baggage from sloppy purification.
Quality slips fast if storage goes wrong. This compound asks for tight, moisture-free packaging, ideally amber glass under inert gas. Desiccators can help out in humid climates. Skipping these basics increases byproducts, threatening every downstream experiment. Every time I’ve stretched a bottle beyond its “best before,” results get noisier, solvents creep up, the whole package starts to degrade. Labs keep detailed logs now, not out of bureaucratic fuss, but because the cost of failed experiments far outpaces the price of proper storage.
Traceability matters. It’s tempting to go cheap, but with critical building blocks like methyl 3-amino-4-methyl-2-thenoate, the origin story becomes everything. Good suppliers deliver an audit trail—batch records, certificates, documented handling—plus clear test results by accredited labs. Companies stepping up now use blockchain-backed systems and QR tracking to minimize the risk of swapped or doctored material.
Buyers should demand transparency, run their own spot checks, and stay alert to any trend in impurity spikes or performance issues. Lessons learned from my own troubleshooting show: put in a little more effort at the point of purchase, and you’ll avoid most chemistry headaches before glassware even hits the bench.
| Names | |
| Preferred IUPAC name | Methyl 3-amino-4-methyl-2-thiophenecarboxylate |
| Other names |
Methyl 3-amino-4-methyl-2-thiophenecarboxylate 3-Amino-4-methyl-2-thiophenecarboxylic acid methyl ester |
| Pronunciation | /ˈmɛθɪl ˈθriː əˈmiːnoʊ ˈfɔːr ˈmɛθɪl tuː θɪˈnəʊ.eɪt/ |
| Identifiers | |
| CAS Number | 37148-47-3 |
| 3D model (JSmol) | `4Z8TPUDEYGFYJJ-NSCUHMNNSA-N` |
| Beilstein Reference | 153735 |
| ChEBI | CHEBI:62018 |
| ChEMBL | CHEMBL186671 |
| ChemSpider | 744833 |
| DrugBank | DB08336 |
| ECHA InfoCard | 100.144.751 |
| EC Number | EC 223-098-1 |
| Gmelin Reference | 78434 |
| KEGG | C19109 |
| MeSH | Methyl 3-Amino-4-Methyl-2-Thenoate |
| PubChem CID | 4460212 |
| RTECS number | KN7160000 |
| UNII | 079TJ2Q56U |
| UN number | UN3334 |
| Properties | |
| Chemical formula | C6H7NO2S |
| Molar mass | 185.23 g/mol |
| Appearance | Light yellow solid |
| Odor | Odorless |
| Density | 1.3 g/cm3 |
| Solubility in water | Slightly soluble in water |
| log P | 0.9 |
| Acidity (pKa) | pKa = 4.53 |
| Basicity (pKb) | 12.08 |
| Refractive index (nD) | 1.580 |
| Dipole moment | 3.59 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 311.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -109.5 kJ/mol |
| Pharmacology | |
| ATC code | This substance does not have an ATC code. |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin irritation, causes serious eye irritation |
| GHS labelling | GHS02,GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302 + H315 + H319 + H335 |
| Precautionary statements | P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 108.5°C (228°F) |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 >5000 mg/kg |
| NIOSH | NA |
| PEL (Permissible) | Not established |
| REL (Recommended) | 30 °C |
| Related compounds | |
| Related compounds |
Methyl 3-Amino-2-Thiophene Carboxylate Methyl 4-Methyl-2-Thiophene Carboxylate 3-Amino-4-Methylthiophene Ethyl 3-Amino-4-Methyl-2-Thiophenecarboxylate 3-Amino-2-Thiophenecarboxylic Acid |