Curiosity about cyclic amino acid derivatives stretches back to early organic chemistry research. Labs in the 1960s plunged into pyrrolidinone compounds, given the explosion of pharmaceutical interest in cognitive enhancers and anticonvulsants. Methyl 2-oxopyrrolidin-1-acetate entered the scene as chemists aimed to tweak the 2-pyrrolidone core, hoping to modulate both solubility and biological interaction. The road to commercial availability ran through Germany and Japan, where innovative researchers knotted together academic curiosity and pharmaceutical demand, paving the way for today’s specialized use across scientific sectors. This historical backdrop means each advancement in synthesis or application draws from decades of practical trial and error, not from theoretical modeling alone.
Often landing in chemical supplier catalogs nestled among specialty reagents, methyl 2-oxopyrrolidin-1-acetate serves as a backbone in the pursuit of new molecules. In practical terms, it’s not the product many folks will find in a household cabinet, but it bridges the worlds of organic chemistry and drug development. Labs use it as a scaffold, before tweaking, coupling, or breaking the structure open for something new. Typically, it’s found in bottles shaded from light, the label clear to avoid any confusion during mixing or weighing in a lab. Demand doesn’t come from everyday hobbyists but from researchers who build on trusted results and predictable performance in reaction setups.
In its pure form, methyl 2-oxopyrrolidin-1-acetate appears as a colorless to faintly yellow crystalline powder, sometimes oily if freshly synthesized. A mild odor hints at its organic roots. Solubility profiles show strong results in ethanol and DMSO, giving chemists flexibility for both reaction planning and bioassay development. Under ambient conditions, it sits stable, resisting hydrolysis for reasonable periods, but it can hydrolyze in strong acid or base. The melting point clusters around 25–30°C, so warm rooms sometimes bring out a hint of stickiness. Molecular weight hovers at 157 g/mol, light enough for fast diffusion but with enough heft to play well in multi-step syntheses. Understanding these characteristics before getting to the lab bench helps avoid costly refrigeration or special shipping, keeping costs manageable.
Specifications aren’t just regulatory niceties. The product needs specified purity levels, with high-performance liquid chromatography (HPLC) checks often cited at 98% or greater before labeling. Common consequences of lower purities include side reactions, wasted solvents, and head-scratching over inconsistent data. Labels usually provide lot number, molecular formula, CAS number, and safety symbols, ensuring traceability for regulators and scientists. In my experience, a clear, accurate label can make the difference between a productive afternoon and hours spent untangling a paperwork mess. Reputable suppliers usually keep their documentation transparent, making it easier for quality control staff to verify that the delivered jar matches what’s on the shelf.
Industrial routes lean on N-acylation of pyrrolidone rings, then methyl-esterification to punch in the acetate group. At the bench scale, stages involve reaction of 2-pyrrolidone with methyl bromoacetate, catalyzed by base, then careful workup to avoid byproducts. Columns packed with silica gel often come out, stripping off impurities. Solvent choice drives success; ether extractions followed by rotary evaporation yield a manageable, purifiable product. Any slip in water control or excess reactant leaves contamination and headaches. These realities give a sense that, while the molecule isn’t wildly exotic, it resists careless scale-up, and consistent quality takes skilled operators with real-world experience in organic synthesis.
Chemists treat methyl 2-oxopyrrolidin-1-acetate like a building block with plenty of adventure left. Standard modifications target the ring or adjacent ester, tapping into acidic or enzymatic hydrolysis, reductive amination, or direct alkylation. Amide bond formation sits high on the list, turning the product into more complex peptide mimetics or heterocyclic systems. Oxygen and nitrogen atoms in the core structure open doors for nucleophilic substitutions, especially for those designing new drug leads. Working with this compound, it becomes clear how predictable reactions can be—if starting materials and procedure are stable, outcomes follow. But any drift in temperature or pH can slip in side products that take work to sort out. Only by paying attention to reaction trends can development scale from milligrams to kilograms.
Across catalogs, this compound can masquerade behind several names, depending on the supplier or historical review. Common synonyms include N-Acetyl-2-pyrrolidone methyl ester, methyl α-(2-oxopyrrolidin-1-yl)acetate, and its IUPAC-longhand, which sometimes sprawls across certificates of analysis. For the uninitiated, these names seem confusing, but accurately matching names prevents ordering the wrong intermediate in a tightly-budgeted project. Sometimes research databases use trade names or abbreviations, so anyone working between European and American suppliers must cross-check CAS numbers against real chemical structures, not just marketing blurbs.
Working safely depends on more than just gloves and lab coats. The Safety Data Sheet spells out the usual routes of exposure—skin, eyes, inhalation. Local regulations frame labeling, and workplace standards often require lockable chemical cabinets, spill kits, and fume hoods for handling. After a minor lab accident forced a mentor to spend two days filling in reports and retraining, the lesson stuck: handling these chemicals means double-checking procedures, avoiding loose containers, and keeping up-to-date training records. GHS pictograms highlight eye and respiratory hazards, but in low quantities, risk can be managed by simple diligence. Drains are off-limits for disposal, and waste must run through the right chemical channels to avoid environmental release.
Drug researchers look at this molecule as a launching pad for nootropics, anticonvulsants, and cognitive boosters. Outside pharma, some agricultural development teams experiment with structural relatives in plant growth studies. This compound’s backbone makes it attractive for ligands in metal-catalyzed reactions or as a precursor to biodegradable polymers. In academic settings, undergrads learn about ester hydrolysis and core functional group interconversion using this and similar molecules. From my time working with early-stage drug candidates, I’ve seen methyl 2-oxopyrrolidin-1-acetate serve as a starting point for adding rings, branching chains, or aromatic groups that shift biological activity in a big way. This adaptability draws in scientists from fields that might never usually overlap—materials, biology, even environmental engineering.
Papers published in medicinal chemistry journals track ongoing tweaks to both synthesis and end-use. Automated synthesis platforms rely on reagents like methyl 2-oxopyrrolidin-1-acetate for high-throughput exploration, feeding directly into machine learning models seeking new therapies. On the bench, researchers hunt for ways to speed synthesis or lower solvent needs as part of sustainability goals. Recent grants support studies on how this compound and its analogues might treat neurodegenerative disease, and academic groups worldwide compete to publish the next technique or application that moves the molecule from “useful intermediate” to “marketable drug.” Progress in scaling production frequently comes from established relationships between chemists and chemical engineers who understand both theoretical needs and practical limitations like solvent cost or yield bottlenecks.
Toxicity studies so far suggest a relatively low acute risk, but chronic effects have not been fully fleshed out. Cell-based assays cover cytotoxicity, but animal studies remain limited. Regulators lean on analogous molecules and commit to conservative exposure limits, especially for staff in pilot plants or research organizations. One of the most challenging aspects is translating in vitro results to real-world human scenarios. Some journals report mild hepatic enzyme elevation in animal models; industry partners call for more long-term observation. Knowing firsthand the gaping holes that still exist in the safety database, most companies emphasize stepwise exposure protocols and regular health checks for anyone coming into daily contact with new compounds.
Looking at industry trends and university pipelines, methyl 2-oxopyrrolidin-1-acetate won’t fade into the background anytime soon. Expansion in both biotech and specialty domains pushes continued demand for reliable sources and new analogues. Automation in research laboratories makes multi-gram batches more common, placing pressure on suppliers to maintain documentation, batch consistency, and cost control. As regulatory landscapes shift regionally, the need for validated toxicity and environmental data grows ever more concrete. Talent in organic synthesis, scale-up, and safety will decide how quickly the molecule moves into new applications—especially as green chemistry principles grow in importance throughout the sector. Success stories from research teams often start with a handful of dependable intermediates, and among them, methyl 2-oxopyrrolidin-1-acetate continues to punch above its weight.
Methyl 2-oxopyrrolidin-1-acetate doesn’t roll easily off the tongue, but this molecule quietly serves in laboratories and pharmaceutical companies around the world. With roots in organic chemistry, it has connections to the family of pyrrolidone derivatives, which turn up in a wide range of research and everyday pharma products. This molecule often turns up in conversations about cognitive enhancers and neuroprotective agents. It’s not as famous as something like aspirin, but in certain scientific circles, it’s a puzzle piece worth knowing.
You’ll see this compound showing up when researchers work on developing drugs for learning and memory. It shares structural fingerprints with substances like piracetam, which scientists and medical professionals have studied for effects on brain function, especially around memory or concentration. Researchers sometimes explore methyl 2-oxopyrrolidin-1-acetate as a starting material for making new compounds—what’s called a chemical intermediate. Its structure gives chemists room to modify or extend, creating novel compounds that get tested in search of better brain medications.
During my time speaking with chemistry professors and industry experts, their respect for these molecular building blocks stood out. Drug development always demands reliable sources of consistent intermediates, so compounds like methyl 2-oxopyrrolidin-1-acetate become the workhorses within the pipeline. Real breakthroughs in medicine can start with these humble molecules.
In addition to being a stepping stone for making other drugs, there’s research into the compound’s potential effects in its own right. Studies sometimes focus on its influence on neurotransmitter activity, particularly on the glutamatergic system, the same system that handles so much of the brain’s signaling and learning ability. Some scientists investigate whether it can be used to protect nerve cells from damage or aging. While no one claims it cures anything on its own, its role as a precursor for new molecules pays dividends over time.
A 2022 review in the European Journal of Medicinal Chemistry highlighted methyl 2-oxopyrrolidin-1-acetate’s part in synthesizing analogues of popular nootropic drugs. Observers of the industry often note how these compounds help researchers generate patentable new medicines, tailored for memory disturbances in dementia or other cognitive issues. The attention stems from real concerns—memory loss affects millions, and safe new therapies lag behind need.
Purity standards matter, especially given how these compounds feed into the pharma supply chain. Laboratories can’t afford sloppy sourcing practices, since cross-contamination or impurities could sabotage experiments or worse, lead to side effects later. Genuine producers work under strict quality management and take safety documentation very seriously.
The regulatory space around these intermediate chemicals continues to change. As with other building blocks for pharmaceuticals, watchdog agencies examine possible diversion to unapproved uses or gray-market supplements. Scientists and policy professionals I’ve met talk about how clear labeling, traceability, and proper export controls all help keep the legitimate industry on track. Education both inside and outside the lab can address remaining blind spots, reducing risks for everyone involved.
While methyl 2-oxopyrrolidin-1-acetate isn’t grabbing national headlines, its behind-the-scenes role keeps labs and innovation moving. Responsible handling, a focus on quality, and constant communication between chemists, regulators, and the public—these will keep feeding progress against the big medical challenges that face us all.
News about unfamiliar chemicals often makes people anxious, especially when complex names like Methyl 2-Oxopyrrolidin-1-Acetate pop up in supplement ads or ingredient lists. As someone who’s spent plenty of time digging through medical studies and keeping an eye on drug development, I see why people ask tough questions whenever these compounds cross into the consumer spotlight.
Methyl 2-Oxopyrrolidin-1-Acetate, sometimes linked with the family of racetams, enters the conversation through channels promising brain-boosting or memory-improving effects. Some users online talk about personal experiences with focus and motivation. None of this replaces careful study. Authority matters—the US Food and Drug Administration, European Medicines Agency, and other respected health organizations set the standard for what’s considered safe to eat, drink, or swallow.
So far, this compound hasn't earned approval for human use by leading regulatory bodies. Digging deeper, reputable databases and published research rarely mention it in the context of controlled trials for humans. Professional chemists can explain its structure and likely behavior, but that doesn’t answer the real worry: “Is it OK for my body?” At this point, scientific documentation looks incomplete.
Plenty of chemicals behave just fine in labs, but things change fast inside the human body. Surprises aren’t rare. Certain nootropics and similar compounds can interact with prescription drugs, trigger allergic responses, or pile stress on the liver and kidneys. Anecdotes floating through online forums or niche supplement sellers can’t stand in for rigorous toxicology and long-term studies. I remember reading about early enthusiasm for some smart drugs that later faded after patient follow-ups showed side effects doctors hadn’t expected.
Consumers need detail. How much gets absorbed? How fast does the body break it down? Does it linger in tissues or flush out quickly? What happens if someone takes other medications? The science takes time—longer than marketers or trend-chasers would like. It’s easy to feel left behind, but cutting corners turns up risks nobody wants.
Curiosity about supplements doesn’t mean throwing caution away. Pharmacy shelves and online shops tempt people with the promise of quick improvements in focus, mood, or mental stamina. Yet the strongest lesson here comes from experience: Always check with a physician or registered dietitian before trying unregulated products. Medical professionals spot risks most of us can’t see. I’ve known folks who assumed a label reading “natural” or “safe” meant they could take anything without thinking twice; sometimes they learned the hard way.
Manufacturers have a duty to fund and share robust research on anything targeting the general public. Until then, buyers have every right to demand clarity. Better education around these compounds helps consumers separate marketing from science. Supporting tighter regulation on supplement labels and pushing for publication of real clinical results protects everyone.
People want answers about what goes into their bodies. Lacking trustworthy evidence, patience and skepticism serve us all better than quick fixes. Communities that stay informed and demand proof over promises help to steer trends in a healthier direction—one substance at a time.
Methyl 2-Oxopyrrolidin-1-Acetate isn’t something most people see every day, but I recognize how much care lab workers pour into handling raw chemical materials. Anyone working with chemicals has felt the sting of an accident, whether it’s a minor spill or a temperature slip-up leading to degraded samples. Even those at home with containers of cleaning supplies can relate—keeping a substance fresh and safe keeps both people and property healthy.
Chemicals like Methyl 2-Oxopyrrolidin-1-Acetate respond to their surroundings. Changes in temperature, exposure to moisture, or simple neglect can lead to unplanned reactions, spoilage, and, at worst, safety risks. Teams put so much effort into conducting reliable experiments or synthesizing products that it only makes sense to protect the quality of their chemicals right from the shelf.
To keep Methyl 2-Oxopyrrolidin-1-Acetate in top condition, a cool, dry spot always trumps a busy, sun-drenched corner of the lab or warehouse. A temperature between 2°C and 8°C fits the bill. Researchers know this isn’t just a random number—the low temperature slows chemical changes and helps the compound keep its structure over time. I’ve seen plenty of labs with specialized refrigerators set aside for these kinds of chemicals. Leaving a bottle at room temperature or in fluctuating conditions increases risk.
Humidity can turn helpers into hazards. A container with a secure, tight cap blocks water vapor from seeping in and stirring up unwanted reactions. Dry surroundings help ensure the powder or liquid inside doesn’t degrade. Labs often stash humidity-indicating desiccants near their stocks for extra insurance. In high school chem class, we learned to pan the room before opening compounds, wiping up nearby spills and checking for hidden leaks.
Direct sunlight always spells trouble, not just for Methyl 2-Oxopyrrolidin-1-Acetate but for a wide array of chemicals. Ultraviolet light can set off slow but steady changes in composition. Storage bottles with brown or opaque walls, tucked away from windows, provide a simple but effective solution. Even experienced chemists tighten lids just a bit more before sliding bottles back on the lowest, darkest shelf.
In my time volunteering in research settings, inventory sheets saved the day. Clear labeling, including date of receipt and expiration, ensures everyone knows what’s safe and ready for use. If containers show any damage—cracks, bulges, or off-color stains—removing and handling them according to hazardous waste guidelines prevents people from getting hurt. Training the team in spill management and emergency responses pays off in the long term.
Tight control over storage spaces makes the difference between a routine day and a scramble to fix something nobody saw coming. Fire authorities and industry regulators expect compliance with chemical storage protocols, not to check boxes but to protect lives. Storing Methyl 2-Oxopyrrolidin-1-Acetate the right way means shielding investments in time, trust, and human safety. Reliable compounds make for reliable results—and that’s always worth the effort.
Most people see “chemical purity” on a label and figure the product does what the box promises. My own years working with lab supplies and household goods taught me that purity rating shapes everything, from whether a compound is safe, to whether it works as intended, and even if it lands on a recall list. Purity is more than a selling point: it’s the backbone of trust in science, industry, and the things we eat, clean with, or use to treat illness.
People think a product labeled “99% pure” sounds great. What they forget is that remaining one percent. That sliver could include dangerous stuff—lead, other heavy metals, or solvents left from manufacturing. The truth is, mistakes in purity have a nasty habit of popping up as public health stories. The contaminated cough syrup incidents in several countries, where toxic impurities slipped through, prove the point. Less talked about, but no less urgent, are household cleaners or supplements that claim a high-purity formulation, only for regular users to get sick from hidden extras no one ever checked for.
Purity starts at the source. Raw materials are packed with impurities by nature. To reach a high-purity product, companies use methods like distillation, crystallization, or chromatography. Each step clears out something unwanted, but these steps cost money and take time. I remember the endless paperwork just to make sure one batch of rubbing alcohol matched the 99.9% mark, because dropping even a tenth of a percent meant it could contain methanol or other unsafe elements.
Even if a factory gets it right, shipping and storage can cause contamination. Leaky containers or poor storage conditions let in dust, moisture, or even plastic from the packaging. It’s not rare for labs to reject shipments thanks to microscopic impurities spotted in a routine test. Good companies trace every drum of raw material, every bottle of finished product, sometimes down to the lot number and the name of a technician who checked it.
Independent labs step in with advanced machines, like mass spectrometers or gas chromatographs, to make sure purity figures aren’t just guesses. Some labs stay open late, working overtime when a pharmaceutical or food recall breaks out, just to run dozens of tests before sunrise. Their reports aren’t perfect, and still sometimes miss rare contaminants, but they keep dangerous products off the market more reliably than any government rule does.
Stories keep rolling in about supplements tainted with unlisted drugs, contaminated flour, or even antifreeze in toothpaste. People suffer, get mad, and demand better. Better means more than stricter rules; it means public pressure for full transparency, right down to the test results. It means customers get to see batch numbers and reports, not just marketing promises.
Anyone who buys chemical-based products can push for this information. Ask for a certificate of analysis, check for third-party testing marks, and treat vague claims with a healthy bit of doubt. Every time someone chooses a more transparent seller, the whole market moves a little closer to making purity mean what it should—safe, trustworthy products for everyone’s home or lab bench.
Anyone working with chemicals for research, product development, or manufacturing knows that trust doesn’t come from bold claims or packaging. It comes from hard evidence that proves what’s in every drum, bottle, or vial. That’s where a Certificate of Analysis (COA) comes in. For a compound like Methyl 2-Oxopyrrolidin-1-Acetate, a COA isn’t just a bonus—it’s a must-have document for anyone who values accuracy and safety.
A COA lists exactly what’s in the material on a chemical level. It spells out purity, impurity profiles, physical appearance, batch numbers, storage recommendations, and more. For someone who’s worked with fine chemicals at bench scale and production scale, I’ve seen how much time, money, and hassle can be saved by checking the COA before moving forward. One time, a batch that looked fine on the surface was flagged as out-of-spec thanks to a simple infrared spectroscopy test. Without a COA, I would’ve had to re-run an entire campaign and eat the cost.
If you’re sourcing Methyl 2-Oxopyrrolidin-1-Acetate for food, pharma, or cosmetics, the paperwork matters just as much as the powder itself. Regulatory bodies, from the FDA in the U.S. to the EMA in Europe, expect clear documentation. They want to see not just a chemical’s name, but its certificate that details exactly what’s been tested and how. Many reputable suppliers post their COAs on request—or even online—so clients don’t waste time chasing paperwork or run the risk of slipping up during inspections. Missing or incomplete paperwork can lead to rejected batches, fines, or even product recalls.
A COA isn’t worth much if the underlying testing is shoddy. Labs backed by ISO certification or Good Manufacturing Practice (GMP) have strict protocols and use methods like HPLC, NMR, and mass spectrometry for purity checks. I’ve run chemical syntheses where a single impurity, invisible to the naked eye, completely changed the way a reaction ran down the line. I once watched a batch of material get pulled off the line because a previously undetected contaminant started messing with a catalyst. Tools like a COA catch those issues before they can turn into bigger problems.
You want suppliers to stand behind their products. Asking for a COA sends a message: “We check our work, and we expect you to do the same.” In my experience, suppliers who hesitate or dodge the request for a COA rarely inspire confidence over the long haul. On the other hand, those who offer batch-specific COAs keep communication open and help you build traceability into your supply chain. If you ever face questions from a client or a regulator, you know exactly where your material came from and how it’s been tested.
Start by making the COA a default part of your purchasing checklist. Make sure you have someone on your team who knows how to read and interpret the technical jargon in these documents—because it means far more than just a stamp of approval. If a supplier refuses or can’t provide one for Methyl 2-Oxopyrrolidin-1-Acetate, find another source. Prioritize suppliers who work with accredited labs and supply detailed, batch-specific documentation. Simple steps like these can make the difference between a project that hits the ground running and one that stalls out before it starts.
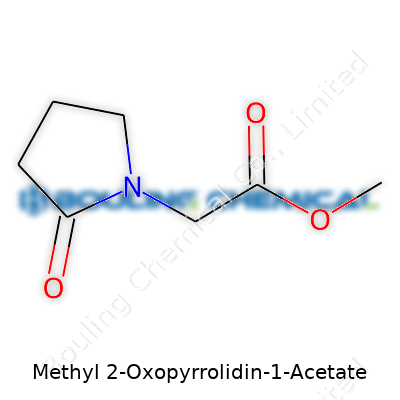
| Names | |
| Preferred IUPAC name | Methyl 2-(2-oxopyrrolidin-1-yl)acetate |
| Other names |
Methyl 2-oxo-1-pyrrolidineacetate Methyl 1-acetyl-2-pyrrolidone-2-carboxylate Methyl 2-oxo-1-pyrrolidineacetate Methyl 2-(1-oxo-2-pyrrolidinyl)acetate |
| Pronunciation | /ˈmɛθɪl tuː ˌɒksəʊpɪˈrɒlɪdɪn wʌn əˈsiːteɪt/ |
| Identifiers | |
| CAS Number | 27347-96-6 |
| 3D model (JSmol) | `JSmol` 3D model string for **Methyl 2-Oxopyrrolidin-1-Acetate** (commonly used as the **SMILES** string): ``` COC(=O)CN1CCCC1=O ``` |
| Beilstein Reference | 119873 |
| ChEBI | CHEBI:65403 |
| ChEMBL | CHEMBL140720 |
| ChemSpider | 185547 |
| DrugBank | DB07045 |
| ECHA InfoCard | 100.096.735 |
| EC Number | EC 255-578-0 |
| Gmelin Reference | 78793 |
| KEGG | C15913 |
| MeSH | D009108 |
| PubChem CID | 89360 |
| RTECS number | UF8583000 |
| UNII | SVR0B9QQVW |
| UN number | UN2811 |
| Properties | |
| Chemical formula | C7H11NO3 |
| Molar mass | 157.17 g/mol |
| Appearance | White to Off-White Solid |
| Odor | Odorless |
| Density | 1.17 g/cm³ |
| Solubility in water | Soluble in water |
| log P | 0.02 |
| Vapor pressure | 0.000197 mmHg at 25°C |
| Acidity (pKa) | pKa = 24.7 |
| Basicity (pKb) | pKb = 7.38 |
| Refractive index (nD) | 1.4800 |
| Viscosity | 1.158 mmHg at 25°C |
| Dipole moment | 4.47 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 322.2 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -743.8 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | N06BX13 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P272, P280, P302+P352, P305+P351+P338, P321, P332+P313, P362+P364, P501 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 100.7°C |
| Autoignition temperature | Autoignition temperature: 385°C |
| Lethal dose or concentration | LD50 (oral, rat): 3200 mg/kg |
| LD50 (median dose) | LD50 (median dose) of Methyl 2-Oxopyrrolidin-1-Acetate: "LD50 (rat, oral) >5000 mg/kg |
| NIOSH | NIOSH: QV5950000 |
| REL (Recommended) | 100 mg |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
N-Methyl-2-pyrrolidone Pyrrolidin-2-one 2-Pyrrolidone Methyl 2-pyrrolidone acetate Ethyl 2-oxopyrrolidine-1-acetate |