People studying amino acids in the early 20th century couldn’t ignore L-Proline for long. Ernst Schulze and his team first uncovered this unique cyclic amino acid from casein. The work felt groundbreaking—an amino acid that didn’t look like any of the others. L-Proline stood out, looping back and bonding to its own backbone, which set scientists off on a journey to untangle the implications for proteins and enzymes. Over the decades, L-Proline moved from novelty to a foundation in biochemistry, medicine, and industry. Researchers began to realize its distinct shape made it crucial in ensuring proteins like collagen built the springy, strong fibers prone to shaping everything from skin to cartilage. The value of proline grew outside of biochemistry, too, winding its way into synthetic processes and forming the backbone of practical applications in pharmaceuticals, food, and research.
Most know L-Proline as a non-essential amino acid, meaning the human body can piece it together from other materials, mostly glutamate. But calling it non-essential hardly does justice to its portfolio. The flavor industry leans on its sweet profile. Food manufacturers list it as a nutrient supplement. Researchers source it for cell culture, while drug companies press it for use in peptide synthesis and chiral catalysis. Each batch rolling off production lines goes to labs, feed manufacturers, or pharmaceutical developers. L-Proline keeps proving that its chemistry isn’t just for biologists—it shapes products that affect diets, treatments, and the development of new therapies.
Pick up pure L-Proline and you’ll find a white crystalline powder, dissolving easily in water but not so much in ethanol or ether. Its structure includes a pyrrolidine ring, locking in a secondary amine and giving it the ability to twist protein shapes. Melting happens around 221-225°C. The unique ring means L-Proline resists forming simple alpha helices, pushing proteins into turns and corners instead. This loop cuts down on protein flexibility, which turns out to be essential for strong, stable structures. The amino acid’s slight taste of sweetness finds use in food flavoring, while its ability to disrupt protein folding is crucial in biological and synthetic experiments.
L-Proline on the international market usually shows a purity level above 98%, often reaching pharmaceutical or analytical grade at over 99%. Typical labeling includes molecular formula (C5H9NO2), molecular weight (115.13 g/mol), and identification through standardized codes like CAS number 147-85-3. Packaging guidelines often cite the need to keep the amino acid dry and shielded from strong light or heat, as moisture and temperature swings can break down its structure. For food or feed applications, legal compliance means adding E-number E170 or specifying it as “L-Proline (Amino Acid)”.
Manufacturers turn to fermentation and sometimes direct chemical synthesis. The fermentation process typically uses Corynebacterium glutamicum or other genetically engineered strains, which can push L-Proline yields while keeping waste down. Extraction then follows, purifying the amino acid through crystallization, filtration, and drying. On a laboratory scale, classic methods such as the Strecker synthesis or enantioselective hydrogenation provide alternative routes, serving cases where small quantities or special isotopically labeled proline are valuable in research. As demand grows, interest in more sustainable, bio-based production continues to rise to avoid problems linked to petrochemical feedstocks.
L-Proline draws chemists in not only because it behaves as a chiral building block but also thanks to its versatility in reactions. Proline acts as both a catalyst and a substrate: it can cyclize peptides, form imines, or reduce carbonyl compounds through enamine formation. In organic synthesis, L-Proline’s enamine mechanism sits at the core of reactions like asymmetric aldol, Mannich, and Michael additions. Biochemists modify proline for deeper protein engineering, using it as a handle to poke and prod at folding pathways or enhance binding affinity. Derivatives such as hydroxyproline appear in collagen and connective tissue research, while esters and N-protected forms help streamline industrial peptide assembly. The chemical reactions involving L-Proline keep multiplying, showing how one small ring can drive a surprising breadth of innovation.
L-Proline carries a family of aliases, reflecting its international reach. Products go under names like (S)-Pyrrolidine-2-carboxylic acid, H-Pro-OH, and Pro. In supplement shops or food labels, you’ll spot it as Prolinum, and sometimes as E170 listed alongside other amino acids. In catalogs catering to drug discovery or research, systematic names dominate: (S)-2-Pyrrolidine carboxylic acid and its enantiomer, D-Proline, underline its chiral significance for chemists. Across language barriers and borders, L-Proline keeps its place as a standard-bearer among amino acids.
Safety standards for handling L-Proline reflect its relatively mild toxicity but recognize that all fine chemicals deserve respect. Workspaces should store it in sealed, dry containers to minimize dust and degradation. Gloves, eye protection, and dust masks go standard in production settings, especially at large scale or when powders could become airborne. Spills clean up with water, but disposal follows regulated waste guidelines to keep contaminants out of water systems. Regulatory reviews, such as those from the FDA and EFSA, continue to clear L-Proline for food and feed usage, okayed as a nutrient additive under typically safe dosage levels. Workers in pharmaceutical labs or factories keep up-to-date through safety data sheets, which specify hazard, storage, and first-aid recommendations tailored by purity grade and intended end use.
L-Proline plays a big role in biomedical research as both a staple amino acid for cell culture and a key in peptide synthesis. Chemists rely on its chiral center to drive advances in asymmetric synthesis, impacting everything from drug development to specialty polymers. In food and beverage production, L-Proline sweetens and fortifies products, supports protein blends in sports nutrition, and steps in as a flavor enhancer or texturizer. The animal feed industry taps L-Proline for poultry and livestock health, improving growth rates and metabolic function. Modern biotech uses it to stabilize enzymes and proteins during formulation.
Many research projects study how L-Proline shapes protein folding and impacts disease, with teams at universities digging into the way substitutions in collagen undermine tissue strength or spark aging diseases. Pharmaceutical firms chase new peptidomimetic drugs that copy proline’s twist, hoping for next-generation treatments for cancer, fibrosis, or even neurodegenerative conditions. L-Proline-catalyzed reactions carve out territory in green chemistry, replacing precious metal catalysts and cutting down on hazardous waste in industrial syntheses. Start-ups and researchers develop bio-based manufacturing, looking for carbon-neutral processes that use renewable feedstocks while keeping yields high and costs under control.
Toxicologists dig deep into L-Proline’s metabolic profile. In mammals, excess intake usually clears safely, with the kidneys handling the routine workload. Yet, studies in rodents and other models flag that abnormally high consumption may disrupt kidney and liver function, trigger mild neurotoxic symptoms, or affect fetal development in rare cases. The food and supplement industries set limits based on those findings, adjusting concentrations to keep finished products within strict safety margins. Allergic reactions or acute toxicity rarely crop up in the published literature, yet ongoing monitoring tracks both worker exposure in manufacturing settings and dietary impacts in end users. The focus remains on long-term intake in vulnerable populations, with regulatory review boards updating daily limits as fresh data arrive.
The story of L-Proline pushes forward as science uncovers new territory in protein design, pharmaceutical development, and eco-friendly chemistry. Research teams plan to redesign enzymes with custom proline substitutions, stepping closer to designer proteins for therapeutics and industrial use. Companies eye bio-based production at scale, searching for methods that cut energy use, shrink carbon emissions, and recycle nutrients. L-Proline’s future in medicine looks promising, as drug researchers turn its unique conformation into anti-cancer strategies, anti-fibrotic therapies, or targeted delivery systems. Green chemistry pioneers explore the limits of proline-catalyzed reactions, aiming for processes that are both cheaper and friendlier to the environment. As innovation continues, more industries will likely find L-Proline indispensable, fueled by both scientific curiosity and the push for sustainable production.
L-Proline isn’t a household name, but it plays a part in things we often take for granted. It’s an amino acid, which means it forms part of the building blocks of protein. Most people don’t think about specific amino acids unless they’re deep into bodybuilding or nutrition science, but L-Proline works behind the scenes everywhere from your skin to your joints.
Proline lives in high amounts in collagen, the stuff that gives structure to skin, cartilage, ligaments, and bones. Collagen isn’t just about smooth skin—though anyone who follows beauty news sees it all over face creams. If you’ve ever recovered slowly from a sprain, or wondered why your skin takes longer to heal with age, collagen comes up as a major reason. Without proline, collagen struggles to form tight, resilient links. Research shows that proline makes up about 17% of collagen’s total amino acid content.
I’ve noticed that as people get older or train hard, they complain about stiff knees and aching joints. Collagen helps those tissues maintain their bounce and flexibility. Proline-rich diets, which include eggs, dairy, and some meats, can support the body’s own collagen production. Studies have observed faster wound closure and softer scar formation in those with adequate proline intake.
Beyond bones and skin, L-Proline may help protect blood vessels from tough plaque and oxidative damage. Proline helps break down proteins for use and repair, preventing the build-up of unhealthy deposits on artery walls. This matters for people with a family history of heart issues, especially as diets have shifted and stress has gone up. The European Food Safety Authority notes proline’s involvement in reducing oxidative stress, which supports healthy vessel function.
Marketers hype up individual supplements all the time. People hear claims about supercharged muscle growth, or miracle cures, which rarely measure up. Proline supplements exist and some users swear by joint or skin benefits, but food sources usually cover daily needs. Adding more eggs, meat, cabbage, or mushrooms helps fill the gaps if your diet feels bland or low in protein.
For those recovering from injuries, surgery, or struggling with chronic wounds, working with a doctor or registered dietitian makes sense before reaching for supplements. Checking overall nutrition—protein, vitamin C, and balanced meals—always beats a magic pill. Vitamin C, in particular, supports the body’s absorption and use of proline. Some hospitals combine vitamin C and proline-rich diets to support patient healing, offering real world evidence for dietary tweaks over expensive capsules.
People who’ve shifted away from heavily processed food often say they feel better all over. I’ve seen relatives get better skin texture and handle winter stiffness with small tweaks, like boiling up bone broths or adding leafy greens. Collagen drinks aren’t always necessary. Common foods can quietly offer all that’s needed. The lesson for anyone interested: look at the ingredients first, focus on what helps the body rebuild naturally, and remember, nutrition builds up slowly—not overnight.
L-Proline stands out as an amino acid that supports healthy joints, connective tissue, and skin. Many people turn to it either for joint comfort after an injury or just to help their bodies keep up with age or athletic demands. Collagen benefits come up a lot when L-Proline gets mentioned, since the body can’t make strong, stretchy collagen without enough of this building block.
Most supplement stores keep capsules or powder forms on their shelves. For adults, the usual supplement dose hovers around 500 mg to 1,000 mg a day. Some labels nudge the numbers up toward 2 grams, sometimes higher, depending on health goals or if a doctor has suggested extra support. It’s always smart to check in with a healthcare provider for advice, especially if taking L-Proline alongside other supplements or prescriptions.
Daily protein from food will include some L-Proline—meat, dairy, eggs, soy, and legumes all supply it. When someone eats a varied diet rich in protein, extra supplementation might not bring major changes unless their health history points to a need.
People often reach for L-Proline powder because it’s easy to measure and mix into shakes, smoothies, or water. I prefer powder myself, since it doesn’t come with fillers you’d find in some capsules. The taste has a slight “amino” bitterness, but it fades in juice or protein drinks. Capsules work well for busy folks who want quick convenience without the fuss of mixing.
Timing doesn’t play a huge part. Many people spread out doses in the morning and afternoon. Since L-Proline isn’t stimulating, there’s little worry about energy swings or trouble sleeping. Stomach upset rarely pops up with these doses, though pairing it with food can help those with sensitive digestion.
Choosing L-Proline with tested purity gives the best chance at real results. Third-party lab testing, clear labeling, and a trusted brand make all the difference. I’ve seen some companies cut corners, using animal-based raw materials without stating the source. Those avoiding animal products can look for “vegan” on the packaging—some new options come from fermented plant sources.
As with any amino supplement, balance matters. It won’t replace all-around nutrition or high-quality protein food. Relying just on one amino acid can tip the scales and, in rare cases, may create imbalances.
High doses could stress kidneys if someone already has kidney troubles. Anyone diagnosed with kidney disease or under treatment should steer clear of self-experimenting. For athletes, runners, and older adults eager to restore tissue, regular check-ins with a doctor or registered dietitian help track progress and avoid surprises.
Reading research before starting a new supplement helps set realistic expectations. Studies point out that extra L-Proline may help joint health, but the evidence grows stronger when combined with vitamin C and full-spectrum protein intake. Rather than chasing a single solution, stacking good habits with smart supplement choices works best.
L-Proline belongs on the shelf mainly for those with joint, tendon, or skin health concerns. For the average healthy eater, food sources usually get the job done. If trying out a supplement, stick close to suggested doses, choose brands with testing, and count on expert advice if underlying health issues exist. No shortcut replaces a balanced approach with regular movement and a good mix of foods every day.
L-Proline stands out as one of the body’s Lego pieces for building collagen, supporting skin, cartilage, and joint health. Some fitness enthusiasts reach for it hoping to recover faster or give their skin a little TLC from the inside out. It’s already found in many foods like egg whites, wheat germ, and dairy, so most folks get their fair share just from a regular diet.
Many supplements promise big things, but every choice comes with trade-offs. For most, adding L-Proline in moderate doses doesn’t kick up major problems. Still, piling on unnecessary amounts can spell trouble. Some people chasing higher doses for muscle gain or anti-aging notice tummy grumbles—think bloating, nausea, or diarrhea. There are stories of headaches and fatigue, too, especially at the start or when splitting high doses throughout the day. These aren’t medical emergencies, but nobody wants to feel lousy for supposed health gains.
Certain health conditions make things trickier. High blood pressure doesn’t mix well with unregulated supplement routines. L-Proline affects how your blood vessels behave, since it’s tied up in building collagen, which keeps arteries strong. There isn’t strong proof that L-Proline alone causes high blood pressure, but studies suggest extra amino acids in supplement form may create unintended shifts.
Anyone with kidney troubles should talk to a doctor first. Too much of any amino acid—including L-Proline—gives sick kidneys a heavier workload. If your kidneys don’t filter out leftovers well, buildup can hit hard over time.
Supplements often get along fine, but the trouble starts when they tangle with prescription meds. No blockbuster studies prove L-Proline clashes with common drugs, yet anyone using medicines for blood pressure or kidney issues needs to let their doctor know about every supplement on the shelf. Sometimes little-known effects sneak up, throwing off the balance your prescriptions are working to create.
Not all supplements are created equal. Some brands push purity and transparency. Others slide through with shoddy ingredient lists or questionable additives. Without strict oversight, contamination is a real risk. Finding a product tested by third-party labs—like USP or NSF Certified—gives peace of mind that you’re not swallowing anything sketchy by accident. I’ve shared a few stories with friends who thought they were doing their bodies a favor, only to realize the supplement they bought online included fillers or contaminants.
Getting L-Proline through food almost never backfires. Overdoing the powder or cap form can create issues even the most dedicated lifter doesn’t want. Your body has natural limits, and there’s no solid evidence backing megadoses for everyday folks. Most research sticks to low or moderate doses, usually a few hundred milligrams up to two grams daily. Anyone tempted to take more because a forum promised magic should weigh short-term gains against the possibility of feeling under the weather or straining their kidneys.
It pays to talk straight with healthcare providers before bringing in any new supplement, including something as common as L-Proline. Trusted advice and careful product choice go a long way. For most healthy adults sticking with food-based sources, risks drop to nearly zero. People with medical conditions or those on medications need deeper conversations. Staying honest about what you’re taking keeps care on target and minimizes nasty surprises down the road.
Folks who pay attention to ingredient lists eventually come across the word “L-Proline.” It’s not as recognizable as protein or vitamins, but for people sticking to vegan or vegetarian diets, it poses big questions. L-Proline is an amino acid, important for collagen production and joint health. Most people don’t just eat it in its pure form, but supplements and processed foods may contain it. The real issue for plant-based diets comes down to one thing: where the L-Proline came from.
Many supplement labels say L-Proline without telling you the actual source. Amino acids in the market can come from either plants or animals. Older production methods used collagen from cow or pig bones—definitely not vegan-friendly. These days, there’s been a huge push for plant-based sources, especially given the rise in demand from vegan consumers. Some brands now make L-Proline by fermenting plant sugars with the help of microbes. This method doesn’t rely on animal tissue.
People switch to vegan and vegetarian diets for reasons that go beyond health. Ethics, the environment, and animal welfare all play a role. The worry about “hidden” animal ingredients is real. L-Proline offers a good example because the same product can come from two drastically different sources. During my years of looking for vegan supplements, I always check not just for “vegan” labels, but for actual evidence that a product uses plant-based microbes or fermentation. Third-party certifications can make a big difference in confidence.
The plant-based alternative isn’t just marketing. There’s research backing up the shift. According to a 2021 industry report, more supplement companies are investing in microbial fermentation because of consumer demand. It fits the bigger trend: consumers are tired of vague answers and want to support companies that align with their values.
Most L-Proline supplements will say nothing about how they’re made. This creates doubt. If you’re vegan or vegetarian, just seeing “L-Proline” isn’t enough. Hunting for the “vegan” logo or certification on packaging matters. If there’s none, direct questions to customer service can lead to answers. From my experience, companies using plant fermentation are usually proud to share it. If you don’t get a clear answer, skip that brand. Plenty of transparent options exist today.
Natural food sources can fill this gap, too. L-Proline is found in soy, asparagus, mushrooms, and cabbage—foods already popular with plant-based eaters. Eating varied whole foods can cover your amino acid needs unless a doctor says supplements are necessary.
The supplement world keeps changing, but labels still leave a lot to be desired. Regulations don’t always force companies to reveal the source of amino acids, so buyers shoulder much of the burden. Reading up, asking questions, and sticking with transparent brands is the best protection right now.
The drive for clarity will likely push more companies into full disclosure and third-party certification. Those who care about the origin of every ingredient build the pressure. I’ve talked to people who care more about their supplement’s backstory than their morning coffee, and with today’s choices, they can have it both ways. L-Proline can be vegan, but only the right brands will prove it.
L-Proline is an amino acid that supports collagen formation, helps with skin healing, and contributes to joint health. Over the past few years, more folks have added it to daily routines, especially anyone working on fitness or dealing with recovery. Some research points toward extra benefits for folks worried about aging or trying to boost athletic performance. Other supplements and medications often end up in the mix. The question pops up a lot in pharmacy waiting rooms and gym locker rooms—can you safely use L-Proline with other products?
Combining supplements, especially amino acids, can cause confusion in the body. The digestive system can only process so much at once. High doses sometimes compete for absorption. Some essential minerals and vitamins, like zinc or magnesium, need careful timing or their action can get blocked. Throwing L-Proline into that scramble brings more variables. L-Proline, by itself, rarely causes side effects. Most healthy people process it just fine. Pairing it with high doses of other amino acids or muscle-growth formulas creates unpredictable results. Some bodybuilders load up on protein blends — in that case, the body might toss out extra L-Proline before it does any good.
More evidence keeps rolling in about supplement and medication interactions. Most research on L-Proline comes from animal studies or small human trials. Scientists haven't flagged major red-alert risks with over-the-counter drugs. Still, certain medications might need special attention. Blood pressure pills, for example: these already influence kidney function, and amino acids like proline could throw off fluid balance if used carelessly. Diabetics face another set of concerns, since some amino acids affect blood sugar levels. Mix them wrong, and numbers may bounce in unpredictable directions. Real-life stories don't always match up with lab data. Some folks on medications for mental health or cholesterol swear by their custom routines. Others tell stories about digestive disruptions or weird muscle cramps after adding new mixes.
I talk with people at the gym and in health forums. Most don’t experience anything dramatic from adding L-Proline, as long as they keep total protein intake reasonable and tell their doctors about changes. The biggest problems show up not from L-Proline itself, but from piling on too many things at once. One friend used L-Proline, magnesium, and a prescription for arthritis — after a month, she started getting headaches, and her doctor realized dehydration and mineral imbalances played a part. Her fix involved better hydration and spacing supplements at different times. Another peer, working through marathon training, stacked L-Proline with branched-chain aminos. Over weeks, he noticed muscle soreness went down, but a blood test showed mild kidney strain. His doctor trimmed back the extras and kept an eye on lab numbers, and things leveled out. Real solutions rarely come in a single pill. Ask questions, get regular checkups, and share honest info with healthcare teams.
No two users are exactly alike. Factors like age, medical diagnosis, diet, genetics, and physical goals change the outcome. Doctors and certified nutritionists remain the best sources for advice, not just marketing labels. High-quality brands can matter—a tablet packed with fillers or unlisted compounds may react differently. Most experts recommend starting slow, writing down any changes—good or bad—and looping physicians into the conversation. Juggling supplements safely means respecting your own limits and keeping a watchful eye on how your body feels. No need to chase trends blindly. Balanced nutrition, trustworthy guidance, and honest conversations with healthcare professionals build stronger foundations than any single supplement ever could.
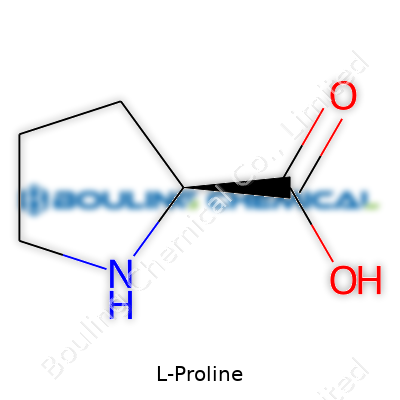
| Names | |
| Preferred IUPAC name | (pyrrolidine-2-carboxylic acid) |
| Other names |
H-Pro-OH L-2-Pyrrolidinecarboxylic acid L-Pyroglutamic acid Proline L(-)-Proline |
| Pronunciation | /ˈelˈproʊliːn/ |
| Identifiers | |
| CAS Number | 147-85-3 |
| Beilstein Reference | 1204560 |
| ChEBI | CHEBI:26271 |
| ChEMBL | CHEMBL780 |
| ChemSpider | 593 |
| DrugBank | DB00172 |
| ECHA InfoCard | 100.004.332 |
| EC Number | 2.3.1.35 |
| Gmelin Reference | 6076 |
| KEGG | C00148 |
| MeSH | D010938 |
| PubChem CID | 614 |
| RTECS number | SY5200000 |
| UNII | 8DNA5V70V8 |
| UN number | UN2811 |
| Properties | |
| Chemical formula | C5H9NO2 |
| Molar mass | 115.13 g/mol |
| Appearance | White crystals or crystalline powder |
| Odor | Odorless |
| Density | 1.35 g/cm³ |
| Solubility in water | Soluble |
| log P | -2.41 |
| Acidity (pKa) | 10.6 |
| Basicity (pKb) | 8.99 |
| Magnetic susceptibility (χ) | -9.72 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.481 |
| Viscosity | 20 cP (20°C) |
| Dipole moment | 4.07 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 86.20 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -528.1 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | −2028 kJ mol⁻¹ |
| Pharmacology | |
| ATC code | A11AA26 |
| Hazards | |
| Main hazards | May cause respiratory tract, eye and skin irritation. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | Glycine;[NH2-]C([H])([H])C([H])(N)C([H])([H])[C@@H](N)C(=O)O |
| Signal word | Warning |
| Hazard statements | No hazard statements. |
| NFPA 704 (fire diamond) | 1-0-0 |
| Autoignition temperature | 455 °C |
| Lethal dose or concentration | LD₅₀ (oral, rat): > 5,000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 >5000 mg/kg |
| NIOSH | Not Listed |
| PEL (Permissible) | 10 mg/m³ |
| REL (Recommended) | 300 mg |
| Related compounds | |
| Related compounds |
S-Proline Prolinol Hydroxyproline Pyrroline-5-carboxylic acid Pipecolic acid |