Isodecyl Diphenyl Phosphate stands as a product shaped by the needs of industrial chemistry and the ongoing search for safer plasticizers and flame retardants. Its origins trace back to the mid-20th century as companies looked beyond phthalates, and scientists realized aromatic phosphates could offer both plasticization and fire resistance. Early adoption stayed mostly within specialized electrical and wire insulation applications, but research in the 1970s and 1980s broadened the stage. Increasing fire safety regulations and concerns around legacy additives drew attention toward organophosphate esters, with isodecyl diphenyl phosphate emerging as a contender for both safety and performance. In practical terms, what matters is that this chemical didn’t drop out of the sky; its development stems from real stakes in industry — both from the desire to meet tighter standards and from recurring pressure to keep workplace and consumer environments safer.
This molecule plays a double role: softening plastics and slowing down fires. You’ll find it mostly in clear, flexible films, electrical cables, and sometimes in furnishings. Its standout feature is that it doesn’t leach or volatilize as quickly as some of its cousins, which already tips the scales toward fewer nuisance odors and lower risk of environmental spread. Because it fits into the broader family of aryl and alkyl phosphate esters, handlers already have a playbook for managing and integrating it in their setups. On a personal note, I’ve seen how shifting to products like this one can ease compliance headaches; big buyers look not just for technical specs but also for something that won’t result in product recalls due to evolving flame retardant standards.
Isodecyl Diphenyl Phosphate shows up as a clear, oily liquid, not all that different at first glance from other industrial plasticizers. What sets it apart is a relatively high boiling point, often nudging 400 degrees Celsius, and a specific gravity sitting near 1.1, so spills look a bit denser than water but not too intimidating for cleaning crews. Its molecular weight lands above 400 g/mol and its viscosity keeps it manageable for batch mixing. Water solubility stays quite low. These characteristics mean hand pumps and drum handlers get along fine, and emissions tend to stay low when the stuff sits in open containers. The chemical structure — a phosphoric acid ester with isodecyl and diphenyl groups — translates into stability in most industrial scenarios; you won’t see it breaking down in a hurry.
Industrial buyers will receive isodecyl diphenyl phosphate with a clear statement on purity — usually above 98% — and tight controls on acidity, color number, and water content. You’ll often see containers labeled with a UN number and a warning icon for “Harmful if swallowed.” SDS sheets call out both acute toxicity and chronic risks. Manufacturers batch test for appearance, refractive index, phosphorus content, and acid value, which helps with transparency when blending with other resin modifiers or flame retardants. Whatever the project size, traceability from batch to batch matters, especially for downstream processors who face audits; clear and accurate labeling isn’t a bureaucratic hoop, but a checkpoint that can save time and trouble down the road.
Making isodecyl diphenyl phosphate demands a reaction between phosphorus oxychloride, phenol, and isodecanol under carefully controlled temperatures with an acid scavenger such as pyridine or a tertiary amine. Batch reactors, corrosion-resistant glass lining, and inert atmosphere are standard to keep the process clean and prevent unwanted byproducts. Overheads vapor shields and process alarms work in tandem for operator safety. The technical recipe relies on stepwise addition of reagents, holding points for mixing and cooling, and post-reaction purification through vacuum stripping or neutralization. Efficiency rises with automation, but hands-on oversight never disappears since reaction conditions change with scale and raw material lot. In real operations, I’ve heard countless stories about how a small calibration error can lead to off-spec batches; meticulous process design makes a real difference.
This phosphate doesn’t just sit idle in finished products. Its chemical backbone allows pairing with other modifiers or reactive monomers, especially in polymer matrices where both plasticity and fire suppression are in demand. During processing at high temperatures, thermal stability holds up to repeated heating cycles, reducing formation of volatile breakdown products. Where additional fire resistance is required, blending with synergists like antimony trioxide or hydrated alumina comes into play. Importantly, the molecule fends off hydrolysis under neutral and mildly acidic conditions, though harsh bases can chew through and yield phenol and isodecanol. At the bench, chemists can tweak chain length or substitute the phenol group to dial performance up or down, which gives R&D teams room to maneuver when performance specs change.
On order forms and in research papers, isodecyl diphenyl phosphate comess under a swirl of names. Some call it IDDP, others stick with its full IUPAC handle: Isodecyl diphenyl phosphate. Commercial brands include Disflamoll DPK, Reofos 50, and Phosflex 41L. In regulatory filings, expect to see CAS Number 29761-21-5. These different names trace back to product lines, regional codes, and the naming habits of chemical suppliers. This sort of jumble matters; I remember colleagues nearly double-ordering when two procurement teams ended up using alternate names for the same drum — a headache that clear communication can nip early.
Handling isodecyl diphenyl phosphate takes more than gloves and goggles. Engineering controls, local exhaust ventilation, and enclosed transfer systems keep vapor and skin exposure low. Prolonged or repeated contact can sting skin and eyes, so direct handling stays under strict lock and key. Fire risk stays low compared with solvent-based additives — the real hazard tends to center on storage temperature and potential for drum leaks. OSHA and EU-REACH frameworks lay out exposure limits and safe work practices, and manufacturers back this up with periodic monitoring and safety audits. In my experience, the companies that follow up on near-misses and actually fix process gaps have far fewer workplace incidents; operational standards only matter if they’re used for more than ticking boxes.
You’ll spot isodecyl diphenyl phosphate in wire and cable jacketing, flexible PVC products, conveyor belts, specialty paints, sealants, and high-end synthetic leather. Auto and aerospace facilities lean on it for durable, burn-resistant upholstery and trim. Furniture makers have switched over to organophosphate blends over old halogenated retardants — a nod both to fire code upgrades and growing consumer concerns. The additive’s performance helps stop flame spread in public venues and transport gear. It’s also a staple in electrical insulation, where performance has to last through years of heating and cooling cycles. What hits home is that real lives get protected when standards embrace substances that work in practice, not just on paper.
Research teams focus on two fronts: pushing the performance envelope and shrinking environmental footprints. Newer studies tackle how this phosphate ester pairs with ecological plastic matrices made from renewable feedstocks, and how breakdown products impact local environments at end of life. Laboratory work across Europe and Asia dives into life-cycle assessments to measure cradle-to-grave effects; some pilot projects propose closed-loop recycling for flame-retarded polymers. The question driving a lot of this is how well the additive holds up once recycled materials get reprocessed again and again. I’ve worked with startups keen to substitute fossil-based raw materials with isodecanol derived from sustainable sources; the difference this makes for ESG scoring can’t be overstated if a company wants access to high-profile retailers.
Published data show moderate acute oral toxicity, but isodecyl diphenyl phosphate doesn’t carry the kind of severe health risks that sent PCBs or PBDEs off the market. Studies in rodents indicate mild liver effects at high doses; workplace exposures rarely come close. Potential for bioaccumulation in aquatic environments stirs regular debate — environmental health scientists look for long-term trends downstream of production and disposal sites. So far, monitoring suggests slow breakdown in soils, but more rapid partitioning to sediments in water. Workers and communities look for independent data, not just company handouts, so credible third-party research helps calm nerves. Eyes stay peeled for any links between chronic low-dose exposure and reproductive health, as this field keeps moving with better tools and larger population studies each year.
Demand is set to remain sturdy, given tightening fire codes and persistent pressure to remove legacy halogenated additives from everything from baby seats to subway cars. Analysts eye a steady rise in both new market sectors and replacement in products with higher performance, especially as manufacturers lean into lifespan, recyclability, and overall environmental footprint. Some regulatory bottlenecks linger, mostly related to aquatic toxicity concerns and persistent lobbying from environmental groups. Many companies have already factored in more stringent disclosure around raw materials and byproducts. If one lesson stands out for future prospects, it’s that success hinges on broad transparency, real data-backed safety, and a willingness to adapt — falling behind in these areas means missing out, not just on profits but on public trust too.
People rarely hear about isodecyl diphenyl phosphate, but almost everyone interacts with products that use it. This chemical often finds its way into the world of plastics and electronics. The story starts in places like old-school factories and ends up in modern homes—right in the palm of your hand, or beneath your feet.
One of the fundamental uses for this compound has to do with fire safety. No one wants a phone, a laptop charger, or the wire casing behind the wall to catch fire at the slightest spark. Manufacturers add isodecyl diphenyl phosphate as a flame retardant to slow down the spread of flames. Decades ago, plastics in electronics burned fast and hot, causing countless home and workplace fires. Getting better flame retardants into the mix made a noticeable difference. According to the National Fire Protection Association, fire safety standards have become stricter since the popularity of synthetic materials surged, and innovations like these chemicals are part of the reason fatalities fell over the past generation.
Beyond flame resistance, this compound also serves as a plasticizer. Think of the wires behind a TV—the reason they’re flexible has a lot to do with chemicals like isodecyl diphenyl phosphate. It softens up PVC, making cables easy to coil but tough enough to handle daily wear. I remember working on a renovation job, snaking wires through tight crawlspaces. Stiff wiring would have turned a full day into a grueling week. These practical improvements matter more than most people realize.
No chemical story comes free of issues. Bits of flame retardants end up in the air, dust, and sometimes even in our food. Research shared by the Environmental Protection Agency points toward some risks if these substances pile up, whether in soil or the food chain. Some studies link phosphate-based flame retardants to negative effects on hormone health in animals. That knowledge should shape decisions by industries and watchdogs. Years ago, folks trusted whatever went into their homes, but now consumers want more answers, transparency, and tighter oversight.
Industry can’t stop using flame retardants entirely—too much safety is tied up in them. Companies and scientists need to push forward with research into safer alternatives that don’t stick around in the body or the environment. More manufacturers have started testing bio-based plasticizers, looking to reduce reliance on older chemicals. Lawmakers in several countries now force companies to list out what goes into everyday products, giving consumers more information and pushing for change.
The story of isodecyl diphenyl phosphate shines a light on a bigger issue: weighing the benefits of safety and performance against risks to health and nature. The best answers come from combining honest science, good manufacturing practices, and clear rules that everyone follows. As a consumer, watching for updates, reading labels, and supporting companies that innovate keeps the pressure on for better, safer options. This is about more than a single chemical—it’s about shaping choices that stretch from the factory floor to everyday life.
Most people haven’t heard of isodecyl diphenyl phosphate unless they work in a field like manufacturing or environmental science. This compound appears in plastics and flame retardants, helping products reach fire safety standards and remain durable over time. If you look at the safety data, you’ll notice that its role keeps growing as industries push for compliance with fire prevention codes. My curiosity grew when I learned that some newer building materials and consumer electronics rely on this additive, which puts it close to daily life.
Health always comes first, especially for parents and vulnerable groups. I’ve seen official agency summaries note that isodecyl diphenyl phosphate doesn’t build up rapidly in animal tissues unlike some older compounds in the same group. Toxicology studies from respected sources, such as the European Chemicals Agency, suggest limited acute toxicity, low skin absorption, and no strong link to mutagenicity (gene changes). At this point, experts generally don’t see high hazard under typical use scenarios.
Despite that, there’s a nagging question about how much we really know. For example, research on long-term exposure still has a few holes. Lab tests can’t fully match what happens in real homes or offices where air, dust, and surfaces mix with human habits. Our daily routines lead not just to breathing in tiny particles but sometimes touching our faces after handling objects containing various additives. The U.S. Environmental Protection Agency has outlined that more research would help clarify possible chronic impacts, especially for small children who might experience higher contact ratios.
I’ve found that people rarely think about slow chemical exposures. Most of us picture “dangerous chemicals” in big spills or factories, not the TV remotes or headphones we use every night. Tiny contributions add up, though, whether through contact or indoor air. My kids often drop toys on the floor and put them in their mouths—something that brings abstract risk a lot closer to real life. If additives like isodecyl diphenyl phosphate migrate out of plastics, even at low rates, the total exposure through dust, hands, or accidental ingestion starts to matter.
Most modern safety standards require companies to keep track of additives and switch them out if credible risk emerges. The EU’s REACH rules, for example, force suppliers to share updated safety profiles. Consumer push-back also changes the game: firms respond quickly if people demand relaxed chemical use, especially if an ingredient faces negative headlines. Replacement chemicals step in—sometimes offering peace of mind but sometimes introducing their own set of unknowns, as I’ve seen in the cases of BPA or flame retardants pulled from children’s products over the past decade.
If you’re worried, there are a few practical steps: wash hands often, especially before eating; keep household dust to a minimum; check labels for certifications like GREENGUARD that suggest tested emission levels; and ask companies for material transparency. No single approach replaces solid scientific review or government oversight, but everyday habits can cut down on unnecessary exposure.
Isodecyl diphenyl phosphate’s safety record so far looks better than many older flame retardants, yet questions remain. Supporting robust research, asking questions, and keeping a clean environment at home go a long way to protect health as science moves forward.
Isodecyl Diphenyl Phosphate stands out as a flame retardant that does its job quietly behind the scenes. Its chemical backbone, forged from an organophosphorus structure, creates a shield against fire. I’ve watched manufacturers trust this compound, especially in plastics and textiles, to buy time when every second matters. According to the American Chemistry Council, phosphate esters like this one remain a top choice for industries focused on reducing flame spread and smoke production. Firefighters and safety inspectors will back this up—materials treated with this phosphate compound react slower to ignition, lowering property risks and improving personal safety.
Durability counts in the real world. Many plasticizers break down, losing performance over the years. Isodecyl Diphenyl Phosphate brings chemical toughness. Heat, sunlight, and humidity pound everyday items, from upholstery to wires, and this phosphate hangs on. It resists hydrolysis, fighting off water damage, and keeps its grip on temperature extremes. The International Journal of Polymer Science found it keeps flexibility intact for plastic products, extending the lifespan of cables and artificial leather.
Nobody wants a product that gives off chemical fumes. This phosphate has a low vapor pressure, meaning it stays put instead of drifting into the air. I’ve seen companies turn to it when they want to limit indoor air quality hazards—especially in places like schools or hospitals. This feature makes maintenance easier; fewer chemicals floating around means less risk and more comfort.
Adapting to new materials puts many additives to the test. Isodecyl Diphenyl Phosphate works smoothly with many types of plastics, including PVC, nitrocellulose, and polyurethane. Formulators prize it for its ability to blend in without causing chemical chaos. Its balance—a mix of rigid aromatic rings and a flexible isodecyl chain—lets it move with the polymer chains rather than fighting against them. That means finished products turn out tough without turning brittle or sticky.
Concerns about pollution and human health keep pressure on chemical suppliers. Phosphate-based compounds bring a safer alternative to halogenated flame retardants, which have faced bans and restrictions worldwide due to their toxic byproducts. A European Chemicals Agency report notes that isodecyl-based phosphates show lower bioaccumulation and better degradation profiles. Still, there’s room for better waste handling and lifecycle planning. Exploring more efficient recycling techniques and developing safer handling protocols would help protect both workers and the local environment.
Materials scientists and regulators bear responsibility for transparency. Full disclosure of chemical risks and open communication with the public matter just as much as technical performance. Regulators should keep pushing for clear labeling and updated safety data sheets, so nobody gets left in the dark. Investors in green chemistry can fund safer synthesis methods that lower waste and reduce emissions, making a real difference by backing practical research and cleaner alternatives.
Most people rarely stop to consider the full backstory of chemicals in household or industrial products. Isodecyl diphenyl phosphate, a plasticizer and flame retardant found in everyday goods like wire insulation and floorings, rarely gets much attention. The shift toward more sustainable living pushes us to look closer at ingredients like these and ask pointed questions about their safety and impact on the environment.
Isodecyl diphenyl phosphate isn’t a natural compound—labs designed it to prevent plastics and rubber from breaking down quickly or catching fire. That seems practical, especially for products where safety and durability matter. Yet synthetic chemicals can linger in landfills, drain into water, and sometimes move up food chains in ways that science still struggles to map.
Research into the environmental behavior of isodecyl diphenyl phosphate paints a mixed picture. According to studies from the European Chemicals Agency, the compound resists breaking apart in water and soil. It clings to sediments and has low solubility, meaning it doesn’t spread out rapidly but doesn’t vanish quickly, either. Over time, that slow breakdown could lead to it sticking around in ecosystems far longer than we’d like.
My work years ago in a municipal water laboratory taught me how plasticizers can slip past basic water treatment. Even those considered less hazardous can accumulate, especially near waste-processing plants or recycling centers. Wildlife ends up picking up trace amounts, and while the immediate damage isn’t always dramatic, the long-term build-up presents real cause for worry.
Many synthetic plasticizers raise red flags for their tendency to build up in living things. Studies on isodecyl diphenyl phosphate show lower bioaccumulation potential compared to older cousins like phthalates. That’s a mark in its favor. Still, it’s not entirely harmless. Chronic exposure in aquatic organisms has led to changes in behavior and growth rates, although the levels needed for this are higher than what common environmental samples exhibit.
Scientists at the U.S. EPA have documented moderate toxicity toward fish and invertebrates after prolonged contact. Wild animals living around manufacturing sites can face higher exposure, which casts doubt on the wisdom of unchecked discharge from factories or improper disposal of flame-retardant goods.
Demand for effective flame retardants isn’t going anywhere. Fire safety laws push companies toward chemicals that slow down burning and reduce toxic smoke. Isodecyl diphenyl phosphate performs well in that respect and has fewer notorious side effects than some alternatives, but calling it “environmentally friendly” overstates the case.
Finding safer options relies on constant research and informed consumer choice. Product manufacturers need real incentives to shift toward greener plasticizers. Stronger regulations on discharge, more thorough recycling efforts, and honest labeling can help keep persistent compounds from piling up in the places we can least afford it. Paying attention to ingredients—even the ones that sound unfamiliar—shapes markets and encourages industry to take environmental stewardship seriously.
Before giving any synthetic additive a pass, it makes sense to keep asking tough questions and demanding better answers from both science and industry.
Open the hood of a car, flip a light switch, power up your laptop—the things we count on every day often depend on specialized chemicals working behind the scenes. Isodecyl diphenyl phosphate pops up in more factories than you might think, despite its name sounding more like a tongue-twister than a power player. Thanks to its ability to resist burning, soften plastics, and keep machines running smooth, this chemical quietly supports plenty of industries that churn out the products we take for granted.
Plastic has shaped modern life, but plastic that can handle heat and not go up in flames? That’s a taller order. PVC (polyvinyl chloride) stretches across industries from food packaging to medical tubing. Additives like isodecyl diphenyl phosphate help keep PVC flexible and, more importantly, make it tough for flames to get hold. Factories making wire insulation, vinyl flooring, and car interiors all keep drums of this chemical on hand. In the world of building and construction, it helps electrical cables meet safety codes, shielding homes and offices from short circuits turning into fire hazards.
Keeping factories and airplanes humming takes more than just basic oils. Many industries use high-performance synthetic fluids that need to work under heavy loads and high temperatures without breaking down. Isodecyl diphenyl phosphate turns up in these fluids as both a flame retardant and plasticizer. Airplane landing gear, power plant turbines, robotics—these systems all lean on this family of additives to keep parts moving smoothly at extremes where other lubricants would burn up. Workers on the shop floor and engineers signing the safety checklist both sleep better knowing the risk of fluid fires stays low.
Electronics factories also use this compound. Think of the circuit boards inside a TV or a phone; fire risk isn’t just about wires. Housing materials, connectors, casings, and even coatings sometimes need extra fire resistance. Isodecyl diphenyl phosphate provides that insurance in places where space is tight and heat has nowhere to go. It’s a bit of a silent partner in making sure short circuits don’t turn into disaster.
The world of coatings and adhesives has shifted too. People expect furniture, office supplies, and automotive interiors to look sharp, feel soft, and last through years of use—all without turning into a safety risk. Flame retardancy often makes the difference between a close call and a tragedy. Adding smart chemicals protects kids’ play rooms, public transport seats, and all sorts of everyday environments. Manufacturers continue to search for safer, better-performing additives, but for now, industry keeps coming back to molecules like this for their reliability and proven results.
Public health demands attention with every chemical added to a product. Regulators keep a close watch on flame retardants, and researchers are chasing safer alternatives with less chance of lingering in the environment or accumulating in living systems. As someone who’s watched safety standards evolve in the manufacturing space, I’m encouraged when companies lean into both effectiveness and long-term sustainability. People want high-performing goods, but not at the expense of air quality or water safety. Open data and clear labeling help both industry and the public track progress—and call out problems early.
In the end, isodecyl diphenyl phosphate doesn’t grab headlines, but its role in keeping the modern world ticking along should not be underestimated. As industries adapt and new materials emerge, the chemistry that makes products safer, smarter, and more durable will keep evolving, shaped by both innovation and real-world needs.
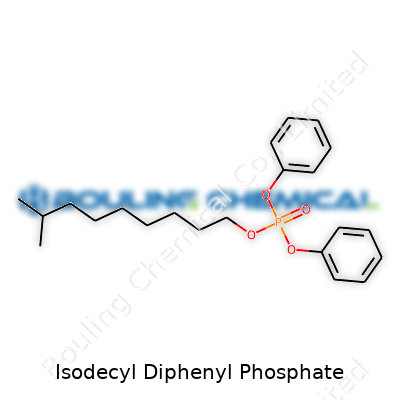
| Names | |
| Preferred IUPAC name | phenyloxy(diphenoxy)decan-6-yl phosphate |
| Other names |
Phosphoric acid, isodecyl diphenyl ester IDPP Isodecyl diphenyl phosphate Diaryl isodecyl phosphate |
| Pronunciation | /ˌaɪ.səˈdɛs.ɪl daɪˈfɛn.ɪl ˈfoʊs.feɪt/ |
| Identifiers | |
| CAS Number | 29761-21-5 |
| Beilstein Reference | Beilstein Reference: 1911124 |
| ChEBI | CHEBI:88043 |
| ChEMBL | CHEMBL557014 |
| ChemSpider | 2333471 |
| DrugBank | DB13919 |
| ECHA InfoCard | 17e1a013-1bf4-45e6-b178-d8bfb1db26d0 |
| EC Number | **29761-21-5** |
| Gmelin Reference | 1445392 |
| KEGG | C19598 |
| MeSH | D010019 |
| PubChem CID | 31504 |
| RTECS number | TD0875000 |
| UNII | N2I3MM8888 |
| UN number | UN3082 |
| Properties | |
| Chemical formula | C22H31O4P |
| Molar mass | 462.57 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Odorless |
| Density | 1.01 g/cm3 |
| Solubility in water | Insoluble |
| log P | 3.8 |
| Vapor pressure | <0.01 mmHg (20°C) |
| Acidity (pKa) | 1.21 |
| Basicity (pKb) | 13.3 |
| Magnetic susceptibility (χ) | \-74.5×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.4850 |
| Viscosity | 13.5 cSt at 25°C |
| Dipole moment | 3.72 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 524.2 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1203.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -13440 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin and eye irritation. May cause respiratory irritation. Toxic to aquatic life with long lasting effects. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H411: Toxic to aquatic life with long lasting effects. |
| Precautionary statements | P210, P273, P501 |
| NFPA 704 (fire diamond) | 1-1-0-0 |
| Flash point | 230°C (Closed cup) |
| Autoignition temperature | 425 °C |
| Lethal dose or concentration | LD50 (oral, rat): > 10,000 mg/kg |
| LD50 (median dose) | LD50 (median dose): >20,000 mg/kg (rabbit, dermal) |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.1 mg/m³ |
| Related compounds | |
| Related compounds |
Tricresyl phosphate Triphenyl phosphate Isodecyl phosphate Cresyl diphenyl phosphate |