The journey of indole began in the 19th century. Adolf Baeyer, a German chemist, described it in the 1860s after isolating it from coal tar and indigo dye. The discovery came during a time when natural products dominated chemical investigation. Scientists soon noticed this ringed structure kept turning up in everything from plant pigments to metabolic byproducts. Over time, chemists figured out ways to make it in the lab. Emil Fischer, another German chemist, unlocked routes that relied on phenylhydrazine and pyruvic acid. Later, synthetic methods grew better with the Fischer indole synthesis, a reaction that shows up in textbooks and on lab benches today. The story of indole isn’t just about the molecule itself — it also tracks the evolution of organic chemistry and the race to understand biology at a molecular level.
Indole pops up in both natural and synthetic worlds. It forms the core for many pharmaceuticals, agrochemicals, flavors, and fragrances. Beyond permeating jasmine and gardenias, it sneaks into animal physiology — think of tryptophan, an amino acid tied to serotonin and melatonin in the brain. Industrial chemists ship indole in volumes large and small, selling to labs, perfumeries, bioengineering companies, and agricultural suppliers.
At room temperature, indole appears as colorless to pale yellow crystals. The scent jumps out — a blend of floral notes and, unmistakably, a faint touch of animal musk. Its melting point sits near 52°C, while the boiling point climbs to about 254°C. Water barely dissolves indole, yet organic solvents like ethanol and ether soak it up with ease. That makes it handy for extractions and downstream purification. The structure, a fusion of benzene and pyrrole rings, gives indole its chemical personality: stability in some situations, but reactive towards acids and bases. It drifts through chemical reactions, tending to favor substitution at the three-position on the pyrrole ring — a feature guiding much of its synthetic chemistry.
Purity stands front-and-center in labeling. Labs seek indole at 98% or higher, since trace impurities muddle results and reduce safety. Common labels note melting and boiling points, structural formula (C8H7N), and batch data for quality tracing. Storage guidance pushes for tightly closed containers, placed away from heat and direct sunlight. Regulatory codes — CAS number 120-72-9, UN shipping designation, hazard pictograms — line up alongside handling advice about gloves, goggles, and ventilation.
Chemists used to lean on natural extraction, boiling pounds of indigo plants for tiny returns. These days, synthetic routes rule. The Fischer indole synthesis leads the pack: arylhydrazines meet ketones or aldehydes, and acids or bases coax the final ring closure. This method unlocks access to basic indole and dozens of analogs — all from affordable starting materials. In the lab, heating the mixture under controlled conditions, then purifying the crude product via distillation or crystallization, gives solid indole with minimal fuss. Industry batches scale up this principle, tweaking catalysts and temperatures for efficiency.
Reacting indole at the three-position brings diverse chemical offspring. Electrophilic substitution drops side chains at this favored spot — a trick used in drug building and dye design. Methylating or acetylating the nitrogen alters solubility, bioavailability, and even scent profiles. Strong oxidizers crack the ring, yielding isatin, another valuable intermediate. Some chemists push further, linking indole to sugars or peptides to mimic natural molecules inside the body. Each tweak opens new doors in pharmaceuticals and materials science, feeding a pipeline of invention and innovation.
People know indole by more than one label. Older research calls it Benzopyrrole or 1H-Indole. Trade suppliers may list Indianole or Ketole. Product catalogs always anchor back to the standard “indole” name, but awareness of these synonyms helps when scanning literature and safety data sheets from different decades.
Looking after yourself comes first. Indole’s crystalline dust can irritate the nose and throat if inhaled. Liquid contact stings the skin and eyes. Industry practice leans on gloves, splash goggles, and fume hoods or solid local exhaust. Storage in cool, dry areas keeps product stable and reduces airborne release. Spills need scooping and careful wipe-downs — not brushing or blowing. Fire considerations factor in, since indole vapor can ignite at the right mix of air and heat. Emergency protocols stick close, and fire suppression gear sits ready. Disposal directs indole waste to licensed chemical incineration or solvent recovery, sidestepping sewer and landfill dumping. These habits keep accidents and exposures rare.
Indole’s chemical backbone touches wide worlds. Drug chemists design antipsychotics, antihypertensives, and anti-inflammatories from indole rings. Bugs and plants build their own versions, using indole signals to fight pests, defend roots, or coax microbes toward growth. Perfume experts dab small amounts into blends, adding a deep richness — floral bouquets lean on that note of indole most people never consciously detect. Agrochemical firms formulate plant hormones and fungal inhibitors, crucial for commercial food supplies. Biologists probe indole pathways to decode brain signaling and gut flora balance. The molecule ties together fields that otherwise might never overlap.
Teams in labs worldwide explore new indole routes, greener syntheses, and novel applications. Chemists test metal catalysts, one-pot strategies, and continuous-flow reactors, aiming for cheaper, cleaner, and faster production. Pharma researchers plug fresh side chains into the indole core, chasing drugs that target cancer, depression, or rare illnesses. Bioengineers engineer microbes to pump out indoles from sugar, skipping harsh chemicals altogether. New detection and purification tools — chromatography, spectroscopy, microfluidic sorters — help these advances move from bench to pilot plant, and, eventually, to the real world.
Indole at modest levels rarely harms healthy people, but high doses or repeated inhalation can knock out the central nervous system and affect liver enzymes. Animal tests point to low acute toxicity — rats swallow grams per kilogram before lethal effects show — yet smaller exposures over weeks depress appetite and activity. The environmental breakdown deserves attention, too. Dumping indole into water or soil lets bacteria munch it down, but intermediate products sometimes linger and stress tiny aquatic creatures. Workplace health studies emphasize air monitoring, personal protective equipment, and ventilation as key to minimizing risks, especially in manufacturing and long-term research settings.
Looking out ahead, prospects for indole shine brightest in green chemistry and medicine. The push for bio-based indole production inspires fermentation processes that swap fossil feedstocks for sustainable crops. New catalysts shrink waste and energy consumption, answering environmental regulation. Drug companies keep mining indole derivatives for therapies that slow tumors and soothe neurological distress. Analytical chemists set their sights on smarter, faster detection of indole — in food spoilage, disease diagnosis, and wastewater treatment. Education and cross-discipline exchange stay vital, since the boundaries of what indole can do keep moving, shaped by fresh insight, new tools, and tighter safety awareness. From perfume counters to hospital labs, this unassuming molecule keeps finding fresh ground to cover.
Some folks hear the word “Indole” and picture a chemistry lab, but its reach goes way further. Indole isn’t just a chemical compound buried in a textbook—it pops up as a backbone ingredient in daily life, research, and industry. Scientists first picked up on its value while analyzing natural substances as far back as the late 1800s, and it’s kept a steady demand since.
It caught my attention many years ago while visiting a crop farm in central India. Farmers there talked about root health as the key to stronger yields. Indole-3-acetic acid, derived from Indole, showed up in soil treatments designed to stimulate plant growth. This compound plays a starring role in boosting natural plant hormones called auxins. Crops treated with these solutions often develop root systems that hold up through heavy winds and drought spells. These aren’t miracle fixes, but they help tilt the odds toward healthy harvests, especially for rice and wheat.
Indole stands out in medical labs for a whole list of reasons. Its derivatives pop up in cancer studies, particularly in testing how certain tumor cells respond. When I worked alongside a cancer research team, I saw how modified Indole molecules helped researchers block some cancer-friendly pathways in petri dishes. Trials are rolling slowly, but these early steps show promise in treatments for breast and colon cancer. On a more routine level, Indole’s fingerprints show up in pharmaceutical formulas ranging from anti-inflammatory pills to some antidepressants.
Most people use Indole without even realizing it. Walk down the detergent aisle: several laundry soaps and cleaning agents blend in traces of Indole for its ability to mask odors and deliver a cleaner, floral scent. It’s also found in perfumes. Synthetic Indole mimics the aroma of jasmine and orange blossoms—two scents that rarely go out of style. Even high-end chocolate brands use small amounts to deepen flavors, especially in dark chocolates where subtlety matters.
Every chemical—natural or not—brings its own risks, though. Indole doesn’t carry the toxic punch of some other compounds, but the agricultural runoff question keeps popping up. Excessive use could disrupt water habitats, especially in places with loose environmental controls. Experts recommend working in batches that match soil needs and using precision farming techniques, like drone surveys, to keep things in check.
More open-source research and field data sharing would help the world tap Indole’s power responsibly. Agricultural extension workers could connect remote farmers to efficient dosing guidelines. In medicine, cross-border collaboration could speed up clinical trials, especially in cancers that respond to Indole derivatives. Regulatory agencies might also keep a closer eye on large-scale industrial use, checking for any unlisted additives or byproducts in food and cosmetic items.
Indole sits at the crossroads of science and everyday need. Its fingerprints touch everything from better soybean yields to advances in chemo drugs. Used carefully and with a dose of common sense, Indole can continue to improve quality of life without pushing environmental or health risks too far.
Indole gets attention thanks to its interesting reputation. You find it in many plants and animal tissues, where it helps shape the smell of jasmine, orange blossoms, and even human sweat. It’s part of tryptophan, an amino acid your body needs. Some folks might brush past stories about indole and just think, “It’s only in a few flowers – what’s the risk?” But this compound lives in more places than perfume. It pops up in tiny amounts in cooked meat, aged cheese, and, believe it or not, even coffee.
People worry about indole because it’s also a byproduct found in the gut after bacteria break down food. Where there’s talk of something unexpected in what we eat, there’s worry. Scientists have dug into the safety question for years. In small amounts, indole doesn’t set off alarm bells for health regulators. The FDA lists it as “Generally Recognized as Safe” (GRAS) for flavoring use, and the European Food Safety Authority agrees, but only at very low concentrations.
Problems start at higher doses. Lab studies using rodents and cell cultures show indole can get toxic if the amount goes way up. High exposure can damage cells and mess with certain organs, but the doses used in research usually dwarf what you’ll find naturally in food or seasoning. Also, natural indole doesn’t hang around alone. In real foods, it teams up with other stuff, which seems to help keep risks low. Physicians look for signs of indole poisoning in people who swallowed big doses by accident, but nearly nobody gets close to those levels through normal diets.
Some diet advice swings from fear to hype, especially when odd-sounding molecules get thrown into the mix. Indole sounds scary if you picture only the lab versions, but forgetting it belongs to the same chemical family as tryptophan misses the point. Skipping cheese or citrus fruits to steer clear of indole cuts out a lot more than this single compound, including nutrients we rely on. Instead, the real work comes from taking a closer look at modern dietary trends and processed products, which sometimes use higher concentrations of additives than what nature intended.
A growing body of research heads toward gut health – the complex relationship between the trillions of bacteria inside us and the chemicals they create, like indole. Some reports suggest that a healthy balance of gut bacteria uses indole and similar compounds as signals, helping the gut barrier stay strong and inflammation in check. Too much or too little changes things, though. People with certain chronic gut issues show unusual indole levels, making it a marker to watch, not a clear villain.
Sticking to balanced eating habits keeps the amount of natural indole low and safe. For people exposed to larger amounts at work, strict safety practices cut down on risks. Food manufacturers in the US and Europe must follow use limits, traceability, and testing rules set by regulators. Researchers continue to test its effects in both humans and animals, working to set safe daily intake benchmarks. Choosing foods made with care, trusting transparent labeling, and supporting efforts to research gut health give people the best shot at safe, smart choices.
Anyone with a rare genetic disorder that affects the breakdown of tryptophan might need to pay more attention; regular people usually don’t face issues if their diet sticks close to natural sources. Taking medical advice matters if you have gut trouble, not just about indole, but about broader food habits. With more research ahead, the relationship between indole, gut health, and overall wellness will keep evolving, but panic over trace amounts in food doesn’t stack up against the health gains of whole food diets and basic moderation.
Indole often pops up in science labs, perfume manufacturing, and even in food flavoring. It’s an organic compound found in coal tar and produced naturally by some plants and the bacteria living in our gut. While its use in the perfume and flavor industry is well documented, indole sometimes finds its way into conversations about health and chemistry for less glamorous reasons.
Few realize how potent indole’s effects can be on the human body. Inhaling or handling pure indole brings out a different side compared to its harmless role in trace food or scent levels. Through personal experience and reports from laboratory staff, handling indole without proper safety measures often leads to skin and eye irritation. People who work with powdered or vapor forms know its smell grows overwhelming fast, sticking to skin, clothing, and any exposed surface.
Acute exposure sometimes brings headaches and a feeling of lightheadedness. The body reacts with nausea or stomach upset if anyone breathes in a moderate dose for too long. Some laboratory workers report these symptoms even before technicians measure significant levels in the air, suggesting sensitivity varies by individual.
Not enough studies have mapped all possible long-term effects of handling indole. Animal tests published in toxicology journals show repeated exposure may affect the liver and kidneys, especially at high concentrations. No one should take this lightly, since chronic low-level exposure can sometimes add up, producing unexpected health challenges after years on the job.
One real example stood out to me: a colleague felt fine for years, then suddenly developed allergic reactions after repeated accidental skin contact with indole solutions. Their doctor suggested that a certain number of exposures had finally tipped the immune system over the edge. Companies that process or manufacture indole often list it as a workplace hazard, offering annual health checks to catch early signs of liver strain or allergic responses.
Aside from direct harm to humans, indole spills can introduce trouble into local water supplies. Even in tiny amounts, indole can act as a pollutant. Studies have shown that watery solutions containing indole disrupt natural aquatic systems, affecting fish and microorganisms. While cleaning up, workers sometimes experience symptoms like skin irritation, even with gloves and masks on.
Wearing the right gear matters more than most people admit. Laboratory safety protocols require nitrile gloves, eye protection, and fume hoods precisely because accidental exposure has long-term consequences. Regular workplace air testing and proper ventilation also help.
Training makes a difference, too. Newcomers in fragrance labs or research environments benefit from hands-on demonstrations. Hearing stories from people who learned the hard way sticks with you much longer than reading another safety sheet. Once someone knows that a small mistake can lead to months of skin rashes, they rarely fail to wash up or ventilate their workspace.
Responsible use of indole calls for transparency from companies and real support for people handling the chemical every day. Solutions include investment in better engineering controls and ready access to healthcare checks. Looking out for one another keeps everyone safer, whether in a busy perfume workshop or a quiet analytical lab.
Indole sits at the crossroads of plant biology and human health. Found in cruciferous vegetables and produced in the gut, this compound pops up on supplement shelves touting everything from hormone support to mood regulation. Many folks want to know how much to take. The problem? There isn’t a one-size-fits-all dosage, and research on supplemental indole remains pretty thin.
Indole itself often comes up in discussions about indole-3-carbinol (I3C) and diindolylmethane (DIM). These are offshoots of indole compounds that pile up in broccoli, Brussels sprouts, and kale. A few studies peek into their effects but often link back to DIM or I3C supplementation, not straight indole. Most available research zeroes in on possible hormone-balancing effects and potential cancer-fighting benefits, but it rarely lays out a clear dosing plan for pure indole. Most supplements set up shop around 200–400 mg for DIM, much higher than any direct indole number because indole is present at microgram levels in food.
No medical authority offers an official indole dosage. With something that comes naturally in everyday foods, people often eat small amounts without thinking. The body breaks down tryptophan (an amino acid) into indole, so all of us ingest a bit just through eating protein. Still, there isn't a mountain of evidence proving that taking extra indole as a pill has clear benefits, and no agency stamps an approval on supplement dosing.
Supplements can surprise folks—one person’s harmless plant extract can play tricks with another person’s health. Reports from the National Center for Complementary and Integrative Health warn against assuming every compound from vegetables automatically makes sense as a capsule. An overload, even of something natural, may stir up unwanted effects in the gut or interact with medications. Too much indole in the body might turn into substances like skatole, which contribute to unpleasant odors and could cause discomfort or other symptoms.
Jumping into supplements without professional guidance often leads to confusion or frustration. Trusted dietitians and doctors look at the full picture—age, existing health issues, and what other pills might be in play. Some folks are more sensitive to things that disrupt estrogen balance, for example, while others chase hormone support for genuinely different reasons. Leaning on advice from healthcare pros, not a stranger from an online forum, offers a much safer route.
Instead of chasing a perfect pill, eating a mix of vegetables does more than most trendy capsules. Piling broccoli, cabbage, and kale on the plate every week brings indole and related compounds the way nature intended, paired with fiber, antioxidants, and other nutrients working in harmony. If a real need for an indole supplement pops up, it makes sense to start with the lowest possible dose, sticking closely to reputable brands, and flagging any changes in mood, skin, or digestion to a professional.
There isn’t a recommended “dose” for indole itself. Any move to take it as a supplement belongs in a conversation with a healthcare provider. Science has just started scratching the surface, and natural options from a balanced diet remain the tried-and-true choice for most people. Erring on the side of caution isn’t old-fashioned—it's smart, and it puts long-term health first.
Indole pops up everywhere, though not everyone gives it much thought. This compound can smell floral at low concentrations, musky or animal-like at higher ones. People in the fragrance world chase it for signature notes. Scientists tinker with it in the lab. Growers see it as a marker in certain crops. All that to say, buying indole is a search that attracts minds from perfume makers to chemists.
Plenty of shops sell indole online. Perfume suppliers such as Perfumer’s Apprentice and The Good Scents Company sell small amounts for creative projects. Ingredient specialty stores like MakingCosmetics or Bulk Apothecary also carry it, often pitched to lab or fragrance mixing customers.
Larger amounts usually mean turning to chemical distributors. Sigma-Aldrich, TCI Chemicals, and Fisher Scientific offer everything from analytical samples to bulk kilos, but you won’t get far without an account tied to a business, school, or research institution. Identity checks and paperwork tend to follow the bigger deals.
Not everyone can buy chemicals on the internet and expect them at their doorstep. If you plan to order pure indole, be prepared to answer questions about end use. Companies like Sigma-Aldrich track sales for safety and regulatory reasons. Customs takes notice of chemical shipments, especially those crossing borders. Some buyers in the United States need to read the DEA’s lists, although indole itself sits outside of restricted status. The main watch-out: using suppliers who don’t bother with safety or standards opens you up to legal headaches.
Indole hooks attention for a simple reason—it’s loaded with possibilities. As the backbone for tryptophan and serotonin, it grounds much of the pharmaceutical world. Agrochemists use it as a plant growth signal. Indie perfumers reach for indole to add depth where flowers feel flat. The risks and hassle make sense given how much hinges on trust in chemical sourcing.
There’s another side to the chase. Low quality or impure indole can ruin a whole perfume batch or skew research results. My own experience with off-brand compounds in a college lab ended in disaster—bad purity wrecked our data. Since then, I only buy from vendors who share safety data and lot analysis.
Check for third-party lab reports or a certificate of analysis. Trustworthy sellers post these upfront. If you’re mixing perfume, ask for customer reviews; experienced noses usually leave feedback on scent, strength, and purity.
Seek out sellers who clearly display company credentials and have established track records. Contact customer service—real people answering basic questions boost confidence. Balanced, transparent sellers protect you from counterfeit or contaminated goods, which in this business, do more harm than good.
Responsible chemistry starts with responsible sourcing. Whether you’re blending rose accords or designing a science project, the right purchase shields both your project and your health.
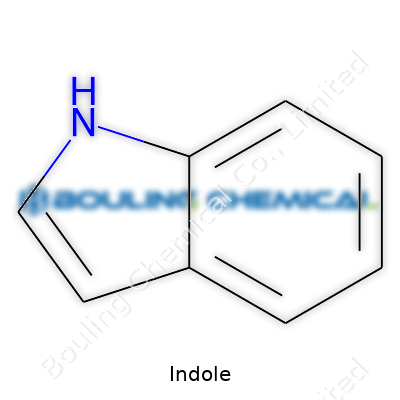
| Names | |
| Preferred IUPAC name | 1H-indole |
| Other names |
Benzopyrrole 1-Benzazole Ketole |
| Pronunciation | /ˈɪn.doʊl/ |
| Identifiers | |
| CAS Number | 120-72-9 |
| Beilstein Reference | 12096 |
| ChEBI | CHEBI:16881 |
| ChEMBL | CHEMBL418 |
| ChemSpider | 700 |
| DrugBank | DB00178 |
| ECHA InfoCard | 100.016.094 |
| EC Number | 3.1.1.92 |
| Gmelin Reference | 82144 |
| KEGG | C00126 |
| MeSH | D007211 |
| PubChem CID | 798 |
| RTECS number | NL2975000 |
| UNII | 83F0R4T84Y |
| UN number | UN2811 |
| Properties | |
| Chemical formula | C8H7N |
| Molar mass | 117.15 g/mol |
| Appearance | White to pale yellow crystalline solid |
| Odor | fecal |
| Density | 1.22 g/cm³ |
| Solubility in water | slightly soluble |
| log P | 2.14 |
| Vapor pressure | 0.016 hPa (25 °C) |
| Acidity (pKa) | 16.97 |
| Basicity (pKb) | -6.95 |
| Magnetic susceptibility (χ) | -79.2·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1. Indole: 1.661 |
| Viscosity | 2.55 mPa·s (20°C) |
| Dipole moment | 2.13 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 111.2 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | +43.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3931 kJ mol⁻¹ |
| Pharmacology | |
| ATC code | N05CM16 |
| Hazards | |
| Main hazards | Causes skin and serious eye irritation. Harmful if swallowed. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS06,GHS08 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P270, P271, P273, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 2-2-0 |
| Flash point | 121°C |
| Autoignition temperature | 410 °C (770 °F; 683 K) |
| Explosive limits | 1.9% - 10.3% |
| Lethal dose or concentration | LD50 (oral, rat): 1000 mg/kg |
| LD50 (median dose) | LD50 (median dose) for Indole: 1000 mg/kg (oral, rat) |
| NIOSH | PY3500000 |
| PEL (Permissible) | PEL (Permissible) of Indole: 3 mg/m³ |
| REL (Recommended) | 3 mg/m³ |
| IDLH (Immediate danger) | Indole IDLH: 750 mg/m3 |
| Related compounds | |
| Related compounds |
Indoline Isatin Indigo Oxindole Carbazole 3-Methylindole |