Imidazolidine’s story reaches back to early explorations into heterocyclic chemistry at the end of the 19th century. Chemists, driven by curiosity and the hunt for new materials, stumbled on the unique five-membered ring structure of imidazolidine compared to related chemical families. The distinct properties of this ring system caught attention in pharmaceuticals and polymers several decades ago, as researchers discovered new ways to use nitrogen-rich compounds to change the performance of drugs and resins. Synthetic routes improved and production could support not only academic work but industrial applications too. The footprint of imidazolidine widened step by step from a curious byproduct to something manufacturers aimed to produce on purpose.
Imidazolidine comes as a colorless, crystalline solid, often produced in bulk for use in laboratories and factories. Its core structure—a five-membered ring containing two nitrogen atoms—sets the stage for consistent chemical reactivity and flexibility. You find it in quality control vials, research jars, and more and more commonly as a base molecule in commercial chemistry. Manufacturers package imidazolidine under various trade names, depending on the modifications and intended market, but the core molecule delivers stable, reliable chemical building blocks.
This compound has a melting point in the moderate range, typically around 130–134°C, with a boiling point well above many common solvents. Its moderate solubility in water and traditional organic solvents, such as ethanol or acetone, makes lab preparation straightforward. The molecule holds up against mild acids and bases, though stronger reagents trigger ring-opening reactions. Its ring system creates a chemical environment both sterically and electronically protected, which is exactly why researchers reach for it during synthesis of pharmaceuticals and polymers.
Material safety sheets list CAS numbers and purity percentages, so researchers and engineers know exactly what enters their experiments. Most commercial imidazolidine comes labeled for purity above 98%, along with recommended storage at dry, cool conditions. Labels list batch numbers for traceability—a step I always appreciate after encountering a few mystery lots during old research internships. Labels carry hazard warnings since the molecule’s high reactivity brings risks on contact or combustion.
Chemists typically start with ethylene diamine and formaldehyde to build the imidazolidine ring. By tweaking reaction conditions, yields climb above 75% in most lab runs. The process uses classical condensation reactions, bubbling off water as a byproduct, and wraps up with recrystallization or distillation. Modern production methods use closed reactors and automated controls, minimizing risks and bumping up consistency batch after batch. The procedures sound routine, but careful temperature control and timing make the difference between a clean run and a sticky mess.
What makes imidazolidine particularly useful lies in its ease of modification. Those two nitrogen atoms aren’t just for show—chemists string on all sorts of side chains to create new derivatives. Halogenation reactions add reactivity, alkylation creates nonpolar analogs, and oxidation steps open the ring for further transformation. In the lab, it often substitutes as a scaffold for ligands or pharmaceutical precursors—handy in synthetic planning for drug discovery. Each lab finds its own favorite routes, but the pattern stays: controlled, reliable, and ripe for new reactions.
Imidazolidine goes by a handful of other names in catalogs and research literature: tetrahydroimidazole, 2,4-dihydro-1H-imidazole, even cyclaminazidine in some old patents. Commercial blends carry branded names with prefixes like “Imido” or “Cyclama,” especially once the molecule bears functionalized side groups or stabilizing agents. The diversity of naming sometimes means having to check chemical structures more than labels—a task that’s tripped up many students during exam prep or literature review.
Working with imidazolidine takes serious attention to personal protection. The molecule, solid as it looks, can cause respiratory irritation if dust escapes. Direct skin or eye contact carries risks, since chemical burns occur in cases of spills or splashes. Proper gloves, goggles, and fume hoods are non-negotiable. Waste disposal runs through hazardous chemical procedures because breakdown products react with environmental moisture and can harm aquatic life. Safety standards base themselves on decades of industrial accidents—no one needs to learn those lessons twice.
The spread of imidazolidine reaches into industrial water treatment, where it acts as a biocide in closed-loop cooling systems. I’ve seen facilities build their entire biophysical controls around this single molecule, since it stops microbial growth without contaminating equipment. Pharmaceutical companies use imidazolidine as a precursor for drugs targeting central nervous system disorders, thanks to its low tendency for metabolic breakdown. In polymer chemistry, it’s a go-to crosslinker or monomer for making heat-resistant materials. Some agricultural researchers modify its skeleton for plant protection products, all with an eye toward safety and environmental persistence.
Academic labs and industrial firms treat imidazolidine as a foundation for building more complex molecules. Newer work explores attaching imaging agents for diagnostic tools, tweaking solubility for intravenous drug delivery, and even making light-activated switches in smart materials. Environmental scientists study its degradation paths, hoping to better understand its role in pollution or natural breakdown cycles. Patents carve out intellectual property around novel uses, which raises the profile and pushes further development. The molecule’s small size and adaptability make it a startup favorite for exploring new chemical spaces.
Imidazolidine demonstrates moderate acute toxicity. Rodent studies indicate LD50 values in the range of 400–700 mg/kg when administered orally. Inhalation tests point to respiratory tract irritation but no long-term carcinogenicity at expected exposure levels. Chronic exposure research remains ongoing, especially with new functionalized derivatives. Occupational health guidelines recommend minimal direct handling and documented exposure monitoring, especially where dust or vapor produces chronic symptoms. Regulators continue to call for biomonitoring evidence, especially after reports linking derivatives to developmental concerns in animal models.
The road ahead looks promising for imidazolidine. Growing interest in sustainable chemistry means researchers are working on greener synthesis routes, pushing for fewer byproducts and milder reagents. Demand in high-tech applications, such as battery electrolytes or anti-corrosion coatings, brings new markets. The biocide role expands where equipment longevity and low secondary pollution matter. Emerging pharmaceutical work on CNS and oncology drugs continues to spotlight imidazolidine as a privileged scaffold. Academic and industrial communities search for modifications that boost safety and bioavailability while reducing environmental persistence. The push-pull between performance and toxicity will keep shaping both lab efforts and regulatory guidelines.
Most folks have never come across the word “Imidazolidine.” If they did, it likely happened on the side of a bottle in tiny print, maybe under the ingredients for some cosmetic or cleaning product. Honestly, it doesn’t sound like much. Yet, this chemical gets tucked away in things we use on our faces and skin, and sometimes right into pharmaceutical formulas.
Imidazolidine works as a kind of “preservative.” Big manufacturers lean on it to keep bacteria and mold out of lotions, shampoos, and even processed foods. It helps stretch the shelf life so their products reach us at the store still looking fresh. After all, nobody wants to dip their hand into a pot of cream and find it crawling with funky growth. Growing up, my grandmother used to keep homemade lotions in the fridge, and they’d start to smell odd after just a week or two. With commercial preservatives, that same lotion could sit out for months without a problem.
What catches many off guard—beyond the makeup aisle—is that imidazolidine also pops up in medicine and industry. Some drugmakers use not just imidazolidine itself, but also its derivatives, to soldier on as building blocks for other chemicals. In the world of plastics and resins, the same ingredient helps finish products we touch every day: kitchen gadgets, car parts, and synthetic rubbers all benefit. For the average person, though, the main place they’ll rub up against imidazolidine is in personal care.
There’s a catch, though. Imidazolidine often releases formaldehyde to do its job as a preservative. Formaldehyde, in small doses, isn’t an immediate danger, but too much can irritate the skin or cause allergies. Sensitive folks might notice breakouts or rashes without knowing the real culprit hiding in the ingredients list. Dermatologists, faced with patients suffering red, itchy skin, sometimes run patch tests and discover imidazolidine-related chemicals at the root. Research groups like the American Contact Dermatitis Society have written about allergic reactions to these sorts of preservatives, so the risk isn’t just theoretical.
Regulators have started paying closer attention. Europe’s health authorities, for instance, have cracked down on the levels allowed in cosmetics. Over the years, I’ve talked to friends who switched to “clean beauty” brands just to avoid stumbling on this very ingredient. Some smaller companies now use plant-based preservatives instead, like rosemary extract or vitamin E oil. These don’t always keep products fresh as long, but the tradeoff sits well with folks wary of skin flare-ups or chemical exposures.
Few people read every word on the back of a product. Still, if you struggle with skin problems, allergies, or unexplained rashes, check for words like “imidazolidinyl urea” or “diazolidinyl urea” inside personal care products. If you notice issues, talk to a dermatologist who actually keeps up with ingredient trends—some dismiss concerns, but others know the common triggers.
Scientists and manufacturers can keep pushing for cleaner alternatives. If enough people ask for it, brands start to listen. Chemical preservatives aren’t going away entirely, but better choices with fewer risks show up when people put up a fuss over what goes into the things they use every day.
People trust medicine with their health every single day. Still, few stop to ask what’s inside the tablet they swallow at breakfast. One ingredient showing up on more chemical lists is imidazolidine. The name sounds straight from a chemistry textbook, and before anyone gets alarmed, it pays to break it down. You might know some of its cousins — many are used as preservatives, releasing a steady drizzle of formaldehyde to kill microbes. The big question: Is this something we should put into our bodies?
Lab folks often like how imidazolidine works in formulas. It keeps medicines stable, fights off bacteria, and stretches out shelf life. This means fewer pills thrown out and lower risk for mold or contamination. That all sounds pretty useful, especially in places where medicines pass through hot warehouses or hang out in humid climates. Shelf stability means sick people might have a fighting chance at getting treatment that hasn't gone bad.
Problems start to creep in with how these chemicals break down. Some imidazolidine derivatives release formaldehyde, which can irritate skin and trigger allergies. In rare cases, folks react with rashes or trouble breathing. The U.S. FDA and European Medicines Agency both set sharp limits on how much of these preservatives can make it into finished drugs or creams. Over the years, research has hinted that regular exposure — especially if you're sensitive — can pile up negative effects. I’ve seen a few concerned friends react to shampoos or lotions packed with similar preservatives, fighting off rashes and irritation they never had before.
There’s a mountain of paperwork behind each ingredient that gets the green light for medicine cabinets or pharmacy shelves. Safety testing covers breakdown products, long-term exposure, and risks for folks who already have allergies or respiratory issues. Yet, problems keep surfacing outside the lab. In 2018, reports of children reacting to topical creams that contained these kinds of preservatives surfaced in Europe. As a parent, those stories stick with me. If smaller or already sick bodies are more at risk, some extra caution sounds overdue.
Regulators keep pushing researchers to come up with safer options that do the same job. Natural preservatives and newer synthetic compounds are showing promise. The pressure is on, especially as more people call out health issues linked to hidden ingredients in everyday products. I keep hearing from pharmacists and compounding specialists who are steering away from imidazolidine and its relatives whenever they can. Alternatives might cost a bit more, but peace of mind is worth it.
Consumers deserve a full picture of what goes into their medicine. Some folks shrug off trace chemicals, others want nothing hidden in fine print. Clear labeling, more public testing, and easier reporting for side effects would all help. If a drug labels itself ‘safe for sensitive skin’ or ‘hypoallergenic’, I want confidence those claims get double-checked. In the end, every ingredient’s safety comes down to honest science, not just what looks tidy on a spreadsheet.
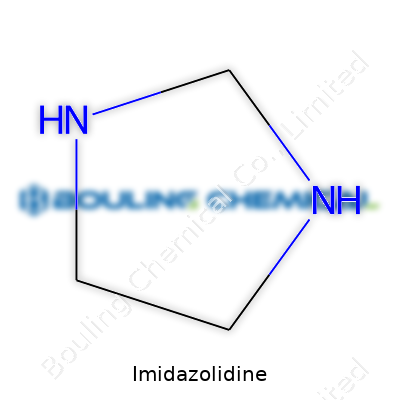
Imidazolidine has a name that sounds right at home in a chemistry textbook, but its value stretches far beyond textbook pages. Shaped like a tiny five-membered ring, this compound pulls hydrogen and nitrogen into a structure that looks simple but packs a punch. A quick glance at its formula—C3H8N2—and you begin to realize this isn’t just any old molecule.
Anyone who’s handled small nitrogen heterocycles will recognize the faintly amine smell drifting off imidazolidine. Most samples come as colorless to pale yellow liquids at room temperature, sometimes showing up as crystals if cool enough. Pour a bit into a beaker and the slick texture reveals its low viscosity. It weighs in at just over one gram per cubic centimeter, floating slightly heavier than water but never quite as thick as syrup. Exposure to the air can turn it a bit brown, hinting at just how reactive it can get outside controlled storage.
Being both basic and nucleophilic, imidazolidine strikes chemists as a versatile player. Those lone pairs on its nitrogen atoms beg to reach out and grab acidic protons or latch onto carbon skeletons during synthesis work. Bring almost any strong acid to the party and you’ll get a salt. Add a drop of strong oxidizer and you risk a runaway reaction—this stuff breaks down in ways that throw off ammonia and a mix of carbon-nitrogen fragments.
React with aldehydes and it cyclizes without fuss, while halogenated compounds yank its electrons around, opening up options for building more complex rings. Not every molecule can step so easily from simple laboratory reactions to the big stage of pharmaceutical or agrochemical creation, but imidazolidine handles that transition with ease.
Water dissolves imidazolidine, thanks to its little polar pockets, but not nearly as well as ethanol or acetone. Its boiling point hovers just above 170°C, which lets you work above room temperature without sweating loss to evaporation. Chemists appreciate that—nothing ruins an experiment quite like your target compound vanishing mid-reaction. Keep this stuff away from strong oxidizers and acids unless you’re ready to clean glassware and fume hoods after an energetic mishap.
Imidazolidine never gained a reputation as a dangerous villain in chemical circles, but that doesn’t mean it’s benign. Breathing in the vapors too long brings irritation, even headaches. Left uncontained, vapors sting the nose and eyes. Gloves and goggles, proper ventilation, and a good fume hood let you avoid most problems.
Disposing of any leftover imidazolidine calls for careful neutralization—dumping it down the drain invites trouble. Following local environmental guidelines prevents groundwater contamination, something that matters as much five years down the road as it does today.
More attention could focus on greener synthesis routes. Right now, industry relies on multi-step processes that generate waste. New catalysts—maybe designed to handle the nitrogen-hydrogen combo with fewer byproducts—could make a real difference. Small improvements at the synthesis stage echo across entire supply chains, cutting costs and shrinking footprints.
Education fits into the puzzle. Sharing hands-on knowledge—how to store, handle, and dispose of imidazolidine safely—keeps newer chemists from learning the hard way. Letting experience guide procedures, rather than just relying on printouts from safety data sheets, builds habits that last a career.
Anyone who’s ever worked around chemicals knows sloppy habits catch up with you. Imidazolidine isn’t just another bottle to shove on a shelf in the back room. Its properties bring some risks, and anyone in a lab, workshop, or plant needs to treat it with a lot of respect. Too many times, I’ve seen corners cut because “it’ll be fine,” and then someone needs to file an incident report or worse.
Every chemistry instructor warns about keeping moisture away from certain chemicals. Imidazolidine fits that list. It’ll break down and might release ammonia odors or trigger unwanted reactions once it picks up enough humidity from the air. So, I always use a desiccator or at least tight, sealed containers. Air-tight jars with screw caps, lined if possible, stop soggy storage and headaches. In my own work, sticking these jars inside a cabinet designated just for low-moisture chemicals solves half the problem.
Light makes some chemicals degrade over time, and imidazolidine isn’t fond of direct sunlight or even harsh lab lighting. I add an amber glass jar and then shut it in the cabinet—just like old tinctures at the pharmacy. It’s simple, cheap, and keeps the stuff stable longer.
Hot rooms speed up chemical changes. Winter storage in a cold warehouse works better than summer, but nobody wants to freeze their stock. In real labs, I keep imidazolidine around 15-25°C, well below the point where anything funny happens. Avoid storage near radiators, heaters, or places with direct sun blasting through the window. In unforgiving climates, climate-controlled cabinets pay for themselves fast. Friends working in tropical regions always curse unreliable air conditioning, but even a steady basement shelf works better than a hot attic.
In shared spaces, one spilled jar can ruin your whole stash. If imidazolidine ends up mixed with acids or oxidizers, the risks compound—sometimes literally. I label everything, use trays underneath each container, and tell coworkers not to stack unlabeled bottles. Anyone who’s mopped up two chemicals gone wrong knows why separate bins and pick-up times matter. Take five seconds to check before returning containers to storage.
Gloves, goggles, lab coat—always. Even if imidazolidine seems benign, repeated exposure adds up. I wear disposable nitrile gloves, not the latex kind, and keep a set of goggles in my locker just for handling dry chemicals. Spills on skin or in the eye aren’t a rite of passage; they send you to the eyewash faster than you’d think. A fume hood isn’t overkill for measuring, especially if you’re pouring or blending larger amounts.
Disposal stumps a lot of people the first time. City trash isn’t an option. Chemical waste pickup once a week prevents forgotten jars piling up under the sink. I use labeled secondary containers for leftover imidazolidine. Waste management pros appreciate clear labeling and dry, sealed bags—less confusion, fewer accidents.
If humidity’s high, silica gel packets inside the outer container work wonders. Poor lighting? Use opaque bins. If space is tight, prioritize keeping reactive chemicals as far apart as possible. For busy labs, training and regular reminders on chemical storage prevent accidents. Write a cheat sheet and tape it inside the cabinet—everyone benefits, new techs especially.
Handling imidazolidine well isn’t complicated, but it demands vigilance. Skipping steps means trouble down the road, often for the person who least expects it. Secure storage, smart handling, and the right gear keep people safe and supplies usable—not a bad trade-off for a little extra care.
Imidazolidine turns up in a surprising number of products. Chemists rely on it for its useful structure. Its derivatives help make preservatives, pharmaceuticals, and even some plastics. Most people never hear the name, yet it plays a role in common items like skin creams or industrial materials. That familiarity breeds a question: is it safe to live with? Experience tells me that small things hidden in long chemical names often carry big risks or rewards.
People usually meet imidazolidine through preservatives like imidazolidinyl urea or diazolidinyl urea. These show up in health and beauty products. Over the years, studies have linked these compounds to skin irritation. Think about using a new lotion and feeling that burning itch soon after. A patch test or two later, the connection often points straight back to those preservatives. The American Contact Dermatitis Society even put some imidazolidine derivatives on its list of top allergens. A 2020 review in the Dermatitis journal reported that up to 3% of adults tested positive for sensitivity to these preservatives.
For folks with sensitive or broken skin, reactions can get worse. Redness, swelling, and painful cracking sometimes follow long-term use. Health professionals see these reactions more often in people with eczema or a history of strong allergies. Others use these products for years without trouble, but I’ve seen friends switch brands after simple rashes that wouldn’t clear up.
Some imidazolidine-based preservatives slowly release formaldehyde over time. That raises another flag. Data from the International Agency for Research on Cancer classifies formaldehyde as a known human carcinogen. Low amounts may not sound risky, yet small daily doses add up, and the health risk can grow, especially for those with constant contact or inhalation. Industrial workers using cleaning solutions containing these preservatives may get exposed much more than the average shopper.
Formaldehyde exposure doesn’t always mean you’ll get cancer—the risks depend on dose and frequency. Short-term effects matter, too. Breathing in fumes or touching formaldehyde-laced materials sometimes causes headaches, coughing, or watery eyes. Some people notice asthma flare-ups. In my experience, once people know where hidden formaldehyde comes from, they seek alternatives fast.
When manufacturers wash down excess imidazolidine or its byproducts into waterways, aquatic life can suffer. Fish and other species may face toxic stress. Regulatory bodies in North America and Europe watch these chemical releases and set discharge limits, but environmental monitoring can fall short. Stories pop up about contaminated rivers, especially near factories, and that damage can ripple far downstream.
Many shoppers now hunt for “preservative-free” or “formaldehyde-free” claims on product labels. Health stores stock alternatives using natural preservatives like potassium sorbate or rosemary extract. Some skincare brands reformulate after hearing customer complaints, and professional groups share lists of safer ingredients. On the worksite, protective gloves, exhaust fans, and strong labeling help reduce direct exposure.
Clearer labeling on product packaging would help, too. People shouldn’t need a chemistry degree to know what’s in their soap. Companies willing to share real ingredient sources win trust and set examples for safer practices. Regulators play a part, but so do consumers willing to ask questions and push for transparency.
| Names | |
| Preferred IUPAC name | imidazolidine |
| Other names |
Hexahydroimidazole Tetrahydroethyleneimine |
| Pronunciation | /ɪˌmɪdəˈzoʊlɪdiːn/ |
| Identifiers | |
| CAS Number | 288-32-4 |
| 3D model (JSmol) | `Imidazolidine|C1NCCNC1` |
| Beilstein Reference | 57359 |
| ChEBI | CHEBI:36611 |
| ChEMBL | CHEMBL14010 |
| ChemSpider | 60724 |
| DrugBank | DB08822 |
| ECHA InfoCard | 23e66a3a-672b-44a5-b0b7-5c6c6f9cbe6c |
| EC Number | EC 232-468-9 |
| Gmelin Reference | 35953 |
| KEGG | C06485 |
| MeSH | D005754 |
| PubChem CID | 8697 |
| RTECS number | NX8925000 |
| UNII | MC3V7F2XV7 |
| UN number | UN3276 |
| CompTox Dashboard (EPA) | DTXSID7066701 |
| Properties | |
| Chemical formula | C3H8N2 |
| Molar mass | 86.136 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.06 g/cm³ |
| Solubility in water | Soluble |
| log P | -0.22 |
| Vapor pressure | 0.32 mmHg (at 25 °C) |
| Acidity (pKa) | 8.6 |
| Basicity (pKb) | 8.62 |
| Magnetic susceptibility (χ) | -76.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.488 |
| Dipole moment | 2.48 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 272.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -62.3 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | −2486 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | N03AX14 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Danger |
| Hazard statements | Harmful if swallowed. Causes serious eye irritation. |
| Precautionary statements | P261, P264, P271, P273, P280, P302+P352, P305+P351+P338, P312, P332+P313, P337+P313 |
| NFPA 704 (fire diamond) | 2-3-1 |
| Flash point | 62 °C |
| Autoignition temperature | 230 °C |
| Explosive limits | Explosive limits: 1.4–7.9% (in air) |
| Lethal dose or concentration | LD50 (oral, rat): 670 mg/kg |
| LD50 (median dose) | 700 mg/kg (oral, rat) |
| NIOSH | SAF40200 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.02 mg/m³ |
| IDLH (Immediate danger) | Not listed. |