Imidazolidine-2-thione’s story traces back to the early explorations of organic sulfur compounds in the nineteenth century. Chemists noticed some unusual behaviors in small heterocycles with both nitrogen and sulfur atoms. Early work focused on unraveling its structure—two nitrogen atoms paired with a sulfur in a five-membered ring. For decades, the compound mostly interested academics. Its importance expanded when industrial labs saw it could serve as a core building block for agricultural chemicals, rubber accelerators, and pharmaceuticals. Increased focus on fine chemicals throughout the twentieth century put imidazolidine-2-thione in the crosshairs of intensive research and commercialization. As manufacturing volume picked up in the latter half of the century, better analytical methods and safer production lines cemented its position in specialty chemical portfolios.
Imidazolidine-2-thione appears as a white to slightly off-white crystalline material. Many in the chemical sector know it under the name 2-mercaptoimidazoline. It’s not flashy, but this powder punches far above its weight thanks to its sulfur-rich character and nitrogen ring. The tangible benefits show up in real-world chemistry. Farmers see its impact as a pesticide intermediate. Rubber technologists appreciate its role as a vulcanization accelerator, supporting faster curing and better product resilience. Pharmaceutical labs keep variants of it close at hand for synthetic campaigns. Each transformation in these sectors often begins with a simple shipment of 2-mercaptoimidazoline from a chemical supplier.
Physically, imidazolidine-2-thione boasts a melting point in the neighborhood of 170°C. It dissolves well in polar solvents—think water, methanol—making it a versatile component in both aqueous and organic synthesis. The crystal lattice remains stable under dry conditions, but excess humidity can affect storage stability. The compound’s distinctive smell, owing to its sulfur group, often signals mishandling or spills. Chemically, the ring system holds up under mild conditions but reacts readily during nucleophilic attacks, allowing for a broad range of functionalizations. The interplay between the nitrogen atoms and sulfur within the ring makes it a prized target for electrophilic substitution or ring-opening transformations.
Standard commercial grades of imidazolidine-2-thione list purity levels above 98%, monitored by HPLC and NMR. Labels include key details: CAS number, molecular formula (C3H6N2S), and safety warnings about skin and respiratory contact. Specification sheets specify thresholds for moisture content, heavy metals, and residual solvents. Packaging focuses on keeping the material dry: plastic-lined drums or foil-sealed cartons prevent contact with air and minimize risks of decomposition. Reliable supply chains stress traceability, so batch numbers, supplier codes, and production dates accompany every shipment. Clear communication helps users avoid confusion with similar, but less reactive, heterocycles.
The most common synthesis route joins ethylenediamine with carbon disulfide under mild alkaline conditions. The reaction produces the heterocycle in a single step. Temperature control and slow addition of starting materials keep side reactions to a minimum. Some labs modify the conditions—warming, using polar solvents, employing phase-transfer catalysts—to lift yields or reduce impurities. After the reaction finishes, crystallization or filtering removes by-products. Recrystallization from water or ethanol polishes the purity. Industrial sites favor processes that bring down costs, minimize waste streams, and recycle excess reagents. Optimized methods have cut reaction times from hours to minutes, which pays off both in safety and overall efficiency.
Imidazolidine-2-thione’s sulfur atom welcomes alkylation, easily picking up methyl or ethyl groups to generate new derivatives. The nitrogen atoms can get acylated, benzylated, or even undergo oxidation. Chemical manufacturers sometimes tweak the ring to fit a particular use, such as linking it to metal complexes in catalysis or pharmaceuticals. You see extensive work on ring expansion or contraction, producing novel analogues for testing in drug studies or material science. Deprotonation of the N-H group allows coupling to more complex organic frameworks. These modifications are not just chemistry for the sake of it; they often lead straight to new commercial products or improved processes in sectors like agriculture and polymers.
Market names and synonyms often pop up in safety data sheets and academic literature. You might see “2-mercaptoimidazoline,” “ethylene thiourea,” or simply “ETU.” Some international suppliers use trade names based on proprietary formulations or blends. Keeping track of synonyms makes a difference in regulatory filings and procurement. Confusing imidazolidine-2-thione with imidazolidinones or other sulfur-heterocycles can cause headaches downstream, from shipping errors to regulatory non-compliance. Most labs train personnel to double-check identifiers against catalog numbers before placing a new order or updating safety inventories.
Direct handling of imidazolidine-2-thione comes with strict rules. Dust inhalation reports have flagged respiratory irritation, and skin contact occasionally sparks sensitization. Storage protocols recommend well-sealed containers, ventilation, and personal protective equipment—gloves, goggles, lab coats. Emergency procedures specify immediate washing with running water and medical checkups for unusual symptoms. Chemical process operators track exposure limits, and industrial hygiene teams monitor ambient air for trace contaminants during handling. Disposal practices follow local hazardous waste legislation, preventing release into water systems or landfill. Some regions require training certificates before site workers can work with the material.
Imidazolidine-2-thione’s fingerprints turn up all over industrial chemistry. In rubber manufacturing, it shortens curing cycles, lending better resilience to tires and hoses without sacrificing strength. Crop protection research uses it for synthesizing fungicides and herbicides, which improves yield and pest resistance without heavy metal residues. Pharmaceuticals benefit from its role as an intermediate for antihypertensives and anti-infectives. Analytical chemistry leans on its ability to chelate metals—crucial in water testing and ore processing. Some researchers even explore its use in corrosion inhibitors, protecting metal structures from decay. The variety of uses makes it a regular in specialty catalogs and a dependable backbone in new product development.
Recent years have seen a surge in projects focused on new derivatives, especially for the agrochemical and medical sectors. Universities and corporate labs invest time and talent into mapping structure-activity relationships, trying to connect tiny shifts in the molecule’s ring to changes in biological or mechanical properties. Research groups have turned to computational modeling, predicting which modifications deliver higher potency or selectivity in crop protection or pharmaceuticals. Pilot plants run small campaigns, verifying that lab discoveries scale safely for commercial rollout. This cycle of discovery, vetting, and production keeps imidazolidine-2-thione at the center of innovation, challenging chemists to push boundaries without crossing lines on safety or sustainability.
Toxicologists approach imidazolidine-2-thione with a measure of caution. High-dose animal studies point to some reproductive and developmental impacts, causing regulatory authorities to monitor workplace exposure closely. Chronic exposure in rubber workers has led to changes in use patterns, and pesticide residues in food crops occasionally show up in regulatory testing. Several countries have set strict occupational exposure limits and push for personal protective equipment in production sites. Continuous toxicological research aims to separate risk from myth, using in-vitro and in-vivo models to pinpoint actual hazards. The compound’s performance gets balanced against safety by regulatory chemists, and periodic reviews adjust acceptable exposure levels as new scientific data emerges.
Imidazolidine-2-thione faces both opportunities and hurdles looking forward. Green chemistry initiatives call for less hazardous production routes—manufacturers continue to test new catalysts and recycling technologies that produce less waste. Regulatory tightening in the mid-2020s put the spotlight on safer handling and alternative compounds in sensitive uses like food packaging and baby products. On the upside, fast-growing applications in smart polymers, controlled-release pesticides, and next-generation drug molecules promise new life for the chemical. The demand for cost-effective, scalable syntheses—coupled with trained chemists ready to tailor-make new derivatives—suggests the compound will keep a strong foothold in research and industry, provided its makers and users stay ahead of the curve on safety and stewardship.
Imidazolidine-2-thione sounds like one of those chemicals that sits tucked away in a dusty textbook, but it actually pops up in some surprising ways. Anyone working in agriculture or food safety runs into this compound. In people’s kitchens, it remains out of sight, but on farms and in labs, it gets a lot more attention than most folks realize. It’s mostly known by its role as a breakdown product of certain pesticides. For those who care about what lands on their food or what lingers in the soil, this compound says a lot.
This molecule shows up after specific pesticides break down. Growers use dithiocarbamate fungicides on crops—think of potatoes, grapes, apples, and other fruits. These fungicides defend against rot and mildew. Once these chemicals break down, imidazolidine-2-thione forms as a residue. Testing for it in produce isn't just a routine step; it marks one of the ways regulators push for food safety. I’ve run residue tests in summer labs, watching scientists hunt for even a trace on a strawberry sample. Reading those chromatograms always reminded me how much behind-the-scenes effort goes into safe produce.
People often hear about fungicide limits and food contamination stories. Government agencies keep an eye on pesticide residues, and imidazolidine-2-thione acts as a marker for dithiocarbamate use. High-performance liquid chromatography, a time-consuming lab method, tracks its levels in food. Regulators use strict thresholds, because, in animal studies, high exposure carried potential health risks, including changes in thyroid function. The numbers don’t lie, so limits put public health above all else. Over the last few decades, those limits have pushed companies to dial back on heavy chemical stacking in fields.
Imidazolidine-2-thione doesn’t just wash away. Rain knocks it into streams and the wider environment. People who care about water quality know that chemicals like this can drift miles from their starting point. Soil scientists and ecologists spend whole seasons studying how molecules leach, break down, or end up in local wells. It’s a reminder, especially for rural communities living alongside big farms, of the invisible line between agricultural progress and environmental responsibility.
Programs like the USDA’s Pesticide Data Program and the EU’s Rapid Alert System for Food and Feed pull data on residues from across the country and continent. Public confidence hinges on this transparency. Sometimes, food lots with higher-than-allowed levels get recalled before reaching shelves. Biological alternatives and innovative fungicide rotations now help keep overall chemical loads lower. On small farms, I’ve seen folks shift away from older chemistry, looking instead to integrated pest management or biopesticides where they can.
Keeping food safe means more than tracking numbers. More research and public sharing of findings let everybody—from growers to consumers—make informed choices. Imidazolidine-2-thione isn’t just a lab curiosity. It’s one small piece in much bigger questions about food, health, and farming’s future.
Most people don’t spend much time thinking about the chemicals in everyday products. I know I didn’t until I started researching what goes into soaps, shampoos, and industrial products. Imidazolidine-2-thione, or 2-mercaptoimidazoline, sounds like something you’d find in a chemistry textbook, far removed from daily routines. Still, it can show up as a preservative or as a reactant in manufacturing processes.
Current research shows that imidazolidine-2-thione can act as a formaldehyde releaser. Even if a product only contains trace amounts, the issue isn’t always about single use—it's about repeated, close contact. Formaldehyde draws concern due to its status as a carcinogen, linked in numerous studies to increased cancer risk after chronic exposure.
Some patch tests performed in dermatology clinics show cases of allergic reactions to imidazolidine derivatives. Redness, itching, or worse—eczema—can develop after skin contact in sensitive individuals. Europe keeps a close watch on preservative safety across cosmetics; regulators often intervene quickly if new evidence emerges showing consistent harm.
Many years ago, I picked up a cheap bottle of hand cream at the drugstore, not thinking much about the ingredient list. A few days later, my skin erupted in red patches. Since then, I watch for formaldehyde releasers in personal care products. I’m not alone. Dermatologists report more people coming in with mysterious rashes, especially after longer use of certain creams and soaps.
The problem with chemicals like imidazolidine-2-thione is that most folks never know what causes the reaction. Labels don’t always mention every byproduct, and the language used isn’t always straightforward. In many cases, someone sensitive to formaldehyde or other preservatives goes through several rounds of trial and error before finding relief.
Countries enforce a patchwork of rules. The European Union restricts certain formaldehyde releasers to low concentrations in skin-contact products. In the United States, oversight falls mainly on manufacturers and consumer reporting. Without robust mandatory labeling, people may expose themselves to irritating or hazardous chemicals without realizing it.
Regulatory agencies base guidelines on long-term studies and reported cases of harm. The challenge always comes down to the lag between scientific discovery and policy. For example, it often takes years of case reports and lab evidence before action picks up on a new risk.
Improved transparency stands out as the single most helpful change. Mandatory ingredient listings, strict labeling for known allergens and formaldehyde releasers, and clear instructions for high-contact products can give consumers tools to protect themselves.
In my own life, I now invest time reading ingredient lists and try fragrance-free, hypoallergenic formulas. For those with chemical sensitivities, patch testing new skincare or cleaning products saves a lot of discomfort down the road.
Better education among manufacturers, professionals, and everyday users brings better health outcomes. Regulators, scientists, and companies must work together to weed out proven harmful substances, and people deserve honesty about what’s inside the bottle. Imidazolidine-2-thione deserves a place in this conversation as science advances and awareness grows.
Growing up in a small town, a lot of my friends’ parents worked in local industries. If you walked into any facility, you could sense that storing chemicals required constant attention. Imidazolidine-2-thione, a compound used in several fields including pharmaceuticals and agriculture, reminds me of those days—chemicals never truly become routine; it's the details that matter.
Scientifically, Imidazolidine-2-thione sits in a league with sulfurous organic compounds, which react to moisture and heat. Long-term exposure to high temperatures or humid air can compromise its stability. According to the European Chemicals Agency, this material stores best at room temperature: 15-25°C, away from any heat sources. Hot storerooms and unregulated warehouses could trigger unwanted chemical reactions, reduce shelf life, or cause the product to clump or degrade. Dry conditions mean less risk for slow breakdown or mold.
Working in quality control, I watched firsthand what happens when humidity seeps into packaging: caked powder, bad batch reports, and wasted hours for staff. Facilities that monitor their air—tracking moisture levels and using silica gel packs—see fewer incidents. An air-conditioned, clean storeroom pays off in fewer losses.
Mixing chemicals out of convenience leads to danger, simple as that. Imidazolidine-2-thione reacts with oxidizers and strong acids. An accidental spill beside bleach or nitric acid can cause a release of dangerous gases. Storage rules in every SDS stress keeping containers away from anything even remotely reactive. At a distribution warehouse I visited, the yellow stickers and separation shelves were not just for show; routine audits caught costly mistakes before they led to emergencies.
Huge accidents rarely happen out of the blue. Most arise from ignoring basic rules. One misplaced drum on a shelf or “just for now” attitude is all it takes. Training staff and enforcing a clear segregation plan should be ongoing, not just a box-ticking exercise at hiring.
Imidazolidine-2-thione wants a sturdy, airtight home. Container integrity affects quality and safety. Steel drums, high-grade plastic, and non-reactive linings beat thin bags or reused containers every time. I remember a case where reused food-grade barrels “seemed fine” but ended up leaking, resulting in a costly recall and hours of cleanup.
Signs and labeling sound obvious, but clear, accurate markings help everyone—especially if you ever have to tackle a spill, inventory check, or audit. Telling one white powder from another isn’t always possible by eye.
A chemical is only as safe as the team handling it. Regular training shifts good intentions into real-world safety. Clean hands, closed lids, sweep up spills—these basics make the difference. Plans for fire or emergency response should include this compound, pointing out any risk for toxic smoke.
Real-world safety goes further than a paper policy. Smart storage protects not only the people inside the facility, but the wider community, the water supply, and the company’s future. Learning from experience, listening to chemical experts, and investing in the right equipment avoids unplanned costs and trouble every time.
Some compounds have structures that sound complex on paper but reveal a lot about function and use when broken down. Imidazolidine-2-thione fits that category. It comes from the imidazolidine family, containing both nitrogen and sulfur atoms arranged in a specific way. This kind of backbone shows up in several important molecules, especially in pharmaceuticals and industrial chemistry.
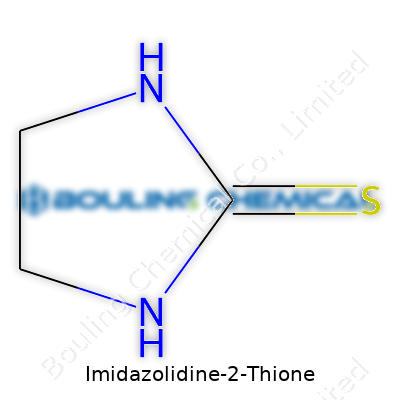
Looking at the name helps. Imidazolidine tells you about a five-membered ring, which means five atoms linked together to form a cycle—two nitrogens, three carbons. The “2-thione” part signals sulfur replacing an oxygen at a certain spot—on the second carbon atom of the ring. This creates a molecule with the formula C3H6N2S.
The backbone sits like this: start with the five-membered ring; place two nitrogen atoms at positions 1 and 3. On position 2, a double-bonded sulfur hangs off, replacing a typical carbonyl oxygen from related compounds like imidazolidinones. This sulfur atom changes the reactivity and the overall electronic nature of the molecule.
An old chemist I studied under used to say, “You can’t talk about use without talking about form.” The arrangement of atoms in imidazolidine-2-thione sets it up for unique interactions—different from a plain carbonyl compound. Sulfur atoms deliver a softer reactivity than oxygen. That switch from oxygen to sulfur (a thione group) impacts both physical properties and chemical behavior, especially in reactions that depend on electron movement, like nucleophilic additions.
This change doesn’t just live in textbooks. The sulfur atom opens doors to form complexes with metals, giving it a role in coordination chemistry and even in some drug designs. Small tweak, big impact. Certain agricultural chemicals and some pharmaceuticals borrow this structure to do jobs that demand stability and selective reactivity, in ways an oxygen version just can’t.
Imidazolidine-2-thione doesn’t sit apart from daily life. In fact, it shows up in studies looking for new medicines and in processes that try to capture or sense metals. Skipping over the details of the shape would miss out on why it’s being studied for both environmental and therapeutic uses. Take the two-nitrogen, one-sulfur combination. That trio of atoms provides a binding site for metal ions, useful for sensors that need to grab onto a target and hold it.
Research has shown this compound’s structure helps bind heavy metals, so scientists are exploring it for use in environmental cleanup. Others experiment with its derivatives as antifungal or antibacterial agents. The differences in activity tie directly to the electronegativity of sulfur and the arrangement of nitrogen atoms. These features change how the molecule interacts in both biological and industrial systems.
The structure of imidazolidine-2-thione raises questions about broader applications and potential side effects in living systems. Detailed studies look at how changing just one atom in a ring affects everything from physical properties to biological activity. The exact placement and identity of atoms like sulfur might seem minor, but history shows these tweaks reshape entire industries.
So, looking at imidazolidine-2-thione isn’t just about memorizing a chemical formula or drawing rings with nitrogens and sulfurs. It’s about following a thread from structure straight through to use, safety, and impact on modern technology and health. Getting to know this molecule’s structure means opening up a toolkit of possibilities—something anyone with a curiosity for chemistry can appreciate.
Imidazolidine-2-thione turns up in a wide range of industrial and laboratory processes, especially for research on organic synthesis or as an intermediate in pharmaceuticals. Many folks new to chemistry don’t realize how easy it is to overlook key steps in handling and disposal. Hazards aren’t just theoretical—skin and eye irritation can happen in seconds, and fumes may leave you coughing for hours if ventilation doesn’t cut it.
My first lesson in dealing with chemicals like imidazolidine-2-thione came early in college. A careless pour into a beaker touched my glove, and a small splash hit my wrist. Despite wearing goggles and using a fume hood, a tiny slip-up led to a lesson about trusting safety protocols, not just equipment. I scrubbed my wrist for five minutes and filled out an incident report—not a badge of honor I wanted but one I never forgot.
Every bottle of imidazolidine-2-thione comes printed with hazard symbols, but familiarity breeds forgetfulness. Control starts with proper gloves—nitrile works best since some solvents chew through latex. Laboratory coats and splash-resistant goggles are essential, not optional. Only work under a certified fume hood where air moves away from your face, taking any stray fumes with it.
Label every container clearly, no matter the quantity. I’ve seen experienced researchers accidentally grab the wrong jar, thinking labels didn’t matter. That’s how chemical confusion starts, and once in the waste bin, there’s no telling what could react with what.
For disposal, mixing imidazolidine-2-thione with regular lab trash or pouring it down the drain can land you or your organization hefty fines—not to mention creating a risk for water supplies. The Environmental Protection Agency places strong restrictions on compounds that may leach sulfur-containing residues into the ground or cause toxicity in local ecosystems. Always collect waste in labeled containers designed for corrosive or toxic organic material, not any old glass jar.
Waste companies often provide these containers at no direct cost, and schedules for pick-up are posted in most research facilities. If your building doesn’t collect hazardous waste frequently, keep the container sealed and away from common work areas until the outgoing shipment day. Never store these containers near food areas—one mistake could lead to accidental contamination.
Creating a safe workplace requires more than following the rules. Encourage questions from newer students or colleagues about safe handling. Host short refresher sessions every semester. Sharing real stories about minor accidents (like my own) makes protocols feel less like paperwork and more like a shared commitment.
Reporting even minor incidents honestly builds trust. It signals that safety isn’t just paperwork; it’s about looking out for one another.
The Occupational Safety and Health Administration (OSHA) offers detailed guidelines on chemical handling, including imidazolidine-2-thione. Material Safety Data Sheets explain in plain language what risks you might face and what to do during exposure. Big universities often upload safety sheets for every chemical in their inventory, so there’s no excuse for not checking one before opening a new bottle.
Investing a few minutes up front—reading the latest guides, double-checking labels, asking for advice—can keep you out of the emergency room and out of trouble with regulators.
| Names | |
| Preferred IUPAC name | imidazolidine-2-thione |
| Other names |
2-Imidazolidinethione Ethylene thiourea ETU |
| Pronunciation | /ɪˌmɪdəˈzoʊlɪˌdiːn tuˈaɪoʊn/ |
| Identifiers | |
| CAS Number | 616-47-7 |
| 3D model (JSmol) | `3DStructure:JSmol|CC1NC(=S)NC1` |
| Beilstein Reference | 87374 |
| ChEBI | CHEBI:35386 |
| ChEMBL | CHEMBL190151 |
| ChemSpider | 21515 |
| DrugBank | DB11217 |
| ECHA InfoCard | ECHA InfoCard 100.007.349 |
| EC Number | EC 206-591-5 |
| Gmelin Reference | 83484 |
| KEGG | C07079 |
| MeSH | D011028 |
| PubChem CID | 69983 |
| RTECS number | MW9620000 |
| UNII | 0476D4G77S |
| UN number | 2811 |
| Properties | |
| Chemical formula | C3H6N2S |
| Molar mass | 104.17 g/mol |
| Appearance | White to off-white crystalline powder |
| Odor | Disagreeable |
| Density | 1.26 g/cm³ |
| Solubility in water | sparingly soluble |
| log P | 0.01 |
| Vapor pressure | 1.91E-05 mmHg at 25°C |
| Acidity (pKa) | 8.1 |
| Basicity (pKb) | 3.47 |
| Magnetic susceptibility (χ) | -69.2·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.740 |
| Dipole moment | 2.97 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 146.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -43.2 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -579.5 kJ/mol |
| Pharmacology | |
| ATC code | D11AX02 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P270, P271, P272, P273, P280, P302+P352, P304+P340, P305+P351+P338, P308+P313, P312, P321, P332+P313, P333+P313, P337+P313, P362+P364, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) | 2-1-0-X |
| Flash point | Flash point: >110°C |
| Lethal dose or concentration | LD50 oral rat 1870 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 97 mg/kg |
| NIOSH | RN 96-45-7 |
| PEL (Permissible) | PEL (Permissible): Not established |
| REL (Recommended) | REL: 0.1 mg/m³ |