Chemists first began exploring the possible uses for imidazole derivatives in the early twentieth century, lured by the unique structure of the heterocyclic ring. Researchers noticed that by bringing bromide ions into the mix with imidazole, it created a salt that sparked interest in labs across Europe and North America. Through decades of synthetic chemistry, the pathway to imidazole hydrobromide moved from unpredictable yields to efficient, reproducible procedures. The compound earned space on the bench thanks to its predictable reactivity, and as biochemistry advanced after World War II, its relevance in catalytic and pharmaceutical research increased. Its story reflects a larger trend in organic chemistry, where practical laboratory methods opened the door for sophisticated biological applications.
Imidazole hydrobromide finds a role in many research labs and production lines as a reliable organic salt, generally appearing as a white to off-white crystalline powder. Its value lies in its function as a source of both imidazole, an aromatic heterocycle, and bromide, a nucleophilic ion used in several organic transformations. In day-to-day work, chemists reach for it because it dissolves well in water and polar solvents, helping reactions proceed smoothly in aqueous and mixed-phase settings. In my own experience, it works with the consistency one expects in pilot-scale synthesis, supporting the intermediate steps in making more complex molecules, especially in the pharmaceutical arena.
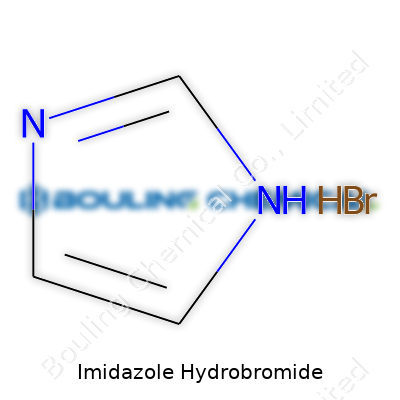
Imidazole hydrobromide’s chemical formula, C3H5N2·HBr, points to its composition—one imidazole molecule paired with hydrobromic acid for the final salt. Melting point typically sits around 165–170°C, with good thermal stability up to that temperature. It dissolves in water at room temperature, and the solution remains clear, pointing to strong solubility. Odorless and non-volatile, the salt resists caking under dry storage, making it easy to handle. The molecule shows moderate acidity, and its bromide ion is chemically active, stepping into substitution and exchange reactions with organic reactants.
Quality standards for this salt go beyond simple chemical purity. For research and industry, reliable labeling specifies not just minimum purity—usually 98% or higher—but also known traces of heavy metals, water content, and the absence of unwanted side-products. Labels should include batch number, date of manufacture, shelf life, handling precautions, and targeted uses, helping end-users make confident decisions. Each batch comes from a documented process, reassuring researchers about reproducibility in their work.
Most batches of imidazole hydrobromide start with imidazole and hydrobromic acid or sodium bromide under controlled conditions. The acid-base reaction between imidazole's nitrogen and hydrobromic acid yields the salt and water as a byproduct. The process involves cooling and precipitation, followed by filtration, drying, and, in cases where even higher purity is required, recrystallization. Facility-grade synthesis places special focus on controlling temperature and mixing speed to avoid side reactions that could introduce impurities—something that can impact sensitive downstream chemistry.
The salt enables a variety of transformations that benefit from both basic and nucleophilic characters. It's often chosen to quench acidic intermediates or introduce imidazole as a reactive moiety in coupling and substitution processes. Pairing with alkyl or aryl halides, imidazole hydrobromide engages in N-alkylation or acylation, supporting synthesis in pharmaceutical drug design or heterocycle expansion. Modifying the compound with additional substituents or using in multicomponent reactions expands its chemical landscape, offering creative routes to new therapeutics and functional materials.
This compound appears in catalogs under a range of names: 1H-Imidazole hydrobromide, Imidazolium bromide, and, less frequently, Imidazole mono hydrobromide. Each label refers back to the same central formula but arises from the supplier’s preferred conventions. International research publications have sometimes muddied the waters by using alternate nomenclature, reflecting the compound’s broad reach and flexible applications.
Handling imidazole hydrobromide involves routine but important precautions. Researchers and technicians need gloves, goggles, and dust masks when working with the powder. Its dust can irritate mucous membranes if inhaled, prompting the need for fume hoods or dust control measures in production lines. Storage away from moisture and strong bases preserves quality, while regular checks for clumping or discoloration help spot degradation. Material safety data calls for immediate rinsing in case of skin contact and prompt medical help if splashed in the eyes. Waste material follows the same regulated disposal as other organic chemicals with halogenated content.
The pharmaceutical sector uses imidazole hydrobromide as a building block for antifungal and antimicrobial agents, benefiting from its capacity to donate or exchange the imidazole ring—a feature in many biologically active structures. Laboratories rely on it for protein purification, where the imidazole structure plays an affinity role in separating proteins tagged with histidine. Other uses spread into dyes and electrochemical devices, where the stability of the salt matches the needs of high-performance materials. In my conversations with industry colleagues, the biggest demand surge always comes from startup biotech companies racing to patent novel enzyme inhibitors or receptor ligands.
Recent years have seen a flurry of R&D work focusing on tailored derivatives of imidazole hydrobromide, targeting cancer therapies and neurological drugs. Researchers push to refine reaction conditions, reduce unwanted byproducts, and uncover new catalytic behaviors. In many cases, development teams seek shorter synthesis pathways and greener chemistry, cutting down on hazardous waste. Collaborative projects between universities and pharmaceutical companies have already produced several patents using imidazole salts as scaffolds for complex bioactive compounds, with major conferences often featuring new abstracts on imidazole-based innovation.
Toxicology teams have run extensive animal and cell-based studies, noting that while the parent imidazole structure can pass through biological membranes with moderate ease, the hydrobromide salt form lowers acute toxicity compared to free imidazole. At practical doses, side effects remain minimal, but higher concentrations introduce mild irritation in sensitive tissue. Regulatory guidance urges careful handling, particularly in early-stage drug development. Recently published papers address potential metabolic breakdown, guidance from regulatory agencies, and risk management processes, suggesting further research for chronic exposure in industrial workers.
Looking forward, the utility of imidazole hydrobromide hinges on advances in material science and drug synthesis. Improvements in production yield, purity, and environmental footprint could open doors for wider use in commercial manufacturing. Custom-tailored imidazole salts stand to reshape part of the antibiotic and anti-inflammatory drug pipeline, prompted by growing resistance to existing medications. Synthetic biologists also explore how these salts can function as molecular “connectors” in assembling artificial enzyme systems. The current trajectory in chemical research and pharmaceutical development keeps imidazole hydrobromide firmly in the toolkit, with the next breakthroughs likely coming from hybrid organic-inorganic applications.
Imidazole Hydrobromide often flies under the radar outside chemistry labs, but ask any scientist working in pharmaceutical development and you’ll hear about its usefulness. This compound, which brings together imidazole and hydrobromic acid, shows up in some crucial places—most of them connected to the way we fight disease, develop new medicines, and study biology on the molecular level. It’s not a blockbuster drug or an ingredient you’ll spot on a shampoo bottle. Most people never encounter it directly, yet its impact stretches much further than its dry, crystalline appearance reveals.
I remember walking through a research lab as a young intern, watching seasoned chemists work with tiny vials labeled with long, intimidating names. Imidazole Hydrobromide was one of those. It acts as a chemical building block, a reagent. Chemists rely on it to make larger, more complicated organic molecules—think antibiotics, antifungals, and enzyme inhibitors. If a scientist wants to explore how a cell works or test a new treatment, they sometimes need to add or remove an imidazole group to a molecule. Imidazole Hydrobromide simplifies these transformations, offering a shortcut in the complex roadmap of organic synthesis.
Bacterial resistance continues to challenge our ability to treat infections. As drug development pushes forward, chemists look for new ways to modify molecular structures. Imidazole Hydrobromide helps build molecules that can slip past bacterial defenses or attach to vital proteins. In this way, it plays a role behind the scenes, supporting the search for medicines that work better or last longer before microbes catch on.
Universities and pharmaceutical companies both use compounds like Imidazole Hydrobromide to test the properties of enzymes and receptors. With a handful of bench reagents, researchers can tinker with molecules, running reactions that highlight why certain medications hit their targets or cause side effects. I’ve seen chemists use it in the early steps of preparing a candidate drug. These experiments can lead directly to clinical trials if all goes well. Even if most compounds created don’t make it to the pharmacy shelf, the data supports everything from safer antibiotics to new cancer drugs.
This compound also gets a nod in fields outside drug research. For example, it turns up in agricultural chemistry, where imidazole derivatives help control fungal diseases in plants. In biochemical analysis, it serves as a buffer or a reactant, letting scientists monitor how proteins fold, change, or break apart.
Like every laboratory chemical, Imidazole Hydrobromide demands respect—both for how it’s handled and where it’s sourced. Reputable suppliers provide documentation because unsafe or contaminated chemicals can set back research and put health at risk. Scientists expect pure chemicals and detailed info about each batch. This trust underpins progress.
The future brings questions, too. Do we always need synthetic reagents, or could biological systems supply alternatives? Researchers keep searching for “greener” chemistry. Some are developing catalysts that reduce waste. Others are mapping out pathways that rely on enzymes instead of harsh chemicals. These efforts might one day shrink the role of compounds like Imidazole Hydrobromide, or they may simply change how they’re produced and used.
Imidazole hydrobromide brings together imidazole—a small, nitrogen-rich ring—and hydrobromic acid. The chemical formula for imidazole itself is C3H4N2. When hydrobromide attaches to this basic nitrogen ring, the formula becomes C3H5N2Br. Each part of this formula represents an element with a specific role. The extra hydrogen hints at the salt that forms after imidazole picks up a proton from hydrobromic acid. In practical lab work, this formula points to a substance you see as a white, crystalline powder—easy to overlook but significant in making reagents and intermediates.
Relying on chemical names without understanding their structure or formula often leads to costly mistakes. Early in my college career, I chalked up an order for a simple compound for a project but ended up with an entirely different salt, slowing work for weeks. That small change in one atom or charged group flips how compounds behave. Imidazole hydrobromide dissolves in water, forms precise hydrogen bonds, and works as a reactant in organic synthesis because of its defined atomic recipe. Getting the formula right isn't nitpicking; it shapes everything from purity checks to regulatory filings and hazard data sheets.
Chemists lean on imidazole derivatives for biology and industry. Drug discovery teams look for structures that mimic the rings found inside DNA bases or enzymes. Biochemistry labs use imidazole salts during the purification of proteins, especially when using the His-tag method. I've watched researchers spend hours troubleshooting a failed separation, only to find the mistake traced back to an impure grade of imidazole salt. That one bromide ion, attached or not, influences solubility, charge, and downstream results.
Even outside research, clear formulas save money in warehouses and shipping docks. Customs rules require declarations based on chemical identities. Staff misreading a formula during packaging can cause delivery delays or trigger unnecessary reports to agencies.
Imidazole hydrobromide isn't hazardous at a glance, but proper labeling and understanding prevent accidents. Over my years in labs, I've seen more bottle mix-ups than actual chemical spills. Busy technicians often scan chemicals by habit, not formula. Standardizing how we list formulas on packaging and in digital catalogs would spare a lot of confusion.
Training newcomers to focus on formulas, not just names, closes most gaps. In classrooms, it helps to see and rewrite real formulas by hand—C3H5N2Br—side by side with similar compounds. Making safety data sheets and online pages more visual, highlighting formulas in bold, reduces the odds of error. Even small changes, such as color-coded labels or interactive online product listings, help newcomers and seasoned staff alike recognize what’s in the bottle.
Imidazole hydrobromide's formula is more than a detail—it underpins reliability from purchase to lab bench. Paying attention here prevents headaches later when projects or safety are on the line.
People expect chemicals to behave the same way every time you use them. That prediction goes straight out the window when storage gets ignored. Imidazole Hydrobromide, a white crystalline powder used in research and synthesis, asks for respect on the shelf. This compound draws water from humid air. Moisture slips in and the structure starts to change—no chemist enjoys that surprise during experiments.
During my time in academic labs, new researchers tried stashing bottles on benches under glaring lights or near heaters. Some wondered why old chemical lots clumped together or lost purity. It never surprised the lab manager. Keeping imidazole hydrobromide dry and cool prevents big headaches down the road. Any slip—humidity, high heat, sunlight—wrecks more than the powder. Suddenly, entire experiment batches fail, wasting budgets, hours, and patience.
Good practice leans on clear instructions. Imidazole hydrobromide should stay in a tightly sealed container to block out damp air. Stash these bottles in a cool spot, out of direct sunlight. Most lab supply firms label 2-8°C as the right temperature range—same as a reliable fridge. Nothing fancy, just protection from the daily swing of warm, muggy air and harsh rays.
Not every stockroom comes with climate control. In warmer climates, chemical cabinets with silica gel packs make a difference. At one facility I visited, the manager lined shelves with moisture-absorbing packets, checked them monthly, and swapped them before any powder could cake. This small detail cut supply losses by half and improved experiment results, according to their lab log.
People who work with imidazole hydrobromide rely on it as a starting material. If it breaks down or develops chunks, mixing it precisely becomes a wild guess. Purity runs down, reproducibility fades, and chemical waste piles up. Even in commercial outfits, margin losses sneak up because it takes more time to analyze, recalibrate, or reorder. Old or poorly stored material can even pose invisible risks—changing reactivity or forming byproducts that threaten the safety of people in the lab.
Two things keep imidazole hydrobromide safe: a habit of checking containers, and using practical storage aids. Clear labeling, regular checks, and up-to-date inventory logs make the difference between reliable supply and costly mistakes. Sealing bottles right after use sounds basic, but I've watched even experienced researchers slip up during busy days.
Labs with bigger budgets install dehumidifiers or small climate-controlled cabinets for sensitive materials. For most, simple steps like double-bagging or adding silica gel keep humidity out. It’s better to spend a few minutes right from the start than to troubleshoot ruined batches later. Some organizations train staff twice a year on chemical storage rules and share real examples of what goes wrong if conditions slide. This helps everyone remember why the rules matter, not just that they exist.
At the end of the day, nobody likes wasted effort. Careful storage cuts down on failures, keeps costs predictable, and lets scientists focus on new discoveries instead of avoidable setbacks. Imidazole hydrobromide proves that even straightforward chemicals show their true value only when handled with a little discipline and respect.
People who work in laboratories or manufacturing sometimes face chemicals that seem obscure to the public. Imidazole hydrobromide is one of those tongue-twisters. It gets used for research, medicine, and industry. Yet, a lot of folks handling it have only a vague sense of what it can do to the body. I’ve watched coworkers underestimate chemicals with complex names, only to regret carelessness later.
No one expects drama from a white powder in a lab jar, but imidazole hydrobromide can stir trouble if mishandled. Breathing dust, touching it with bare skin, or accidentally getting a taste are the main ways people risk exposure. The compound’s structure makes it more irritating than plain table salt or sand. From published safety reports, skin or eye contact brings burning and redness. Getting the dust in your lungs pastes the membranes with a substance that doesn’t belong there. Soon, you cough, your chest tightens, and sneezing fits can last for hours. Prolonged exposure could lead to more lasting respiratory complaints.
I spent time in organic chemistry labs where rules about gloves and goggles weren’t just for show. After seeing a student panic from splashing a similar compound in her eyes, I respect the warnings. Long sleeves, eye protection, and real ventilation are steps that help keep curiosity from turning into a medical emergency.
The short answer? Moderately. It isn’t the most sinister compound in the stockroom, but swallowing, inhaling, or absorbing enough can bring nausea, stomach upset, dizziness, or headaches. LD50 data—the dose needed to kill half a test population—sits above that of cyanide or mercury, yet the risks ramp up for workers exposed every shift. Even low-level chronic exposure hasn’t been well studied in people. If you listen to warnings from the CDC, they make it clear: Unprotected exposure isn’t smart. Symptoms build slowly. Long-term effects sometimes creep in before anyone realizes the link.
Most labs neutralize and dispose of chemical waste in controlled ways, but leaks and spills remain possible. Imidazole hydrobromide is water-soluble, letting it slip through drains and into the environment if protocols aren’t followed. While soil organisms and wildlife might break it down, no one wants extra synthetic chemicals trickling into drinking water. As regulations tighten, facilities face more scrutiny over discharge. Fines for environmental lapses make even minor leaks a major headache. Companies invest in better storage, staff training, and emergency drills. These steps cost money up front but save a lot more in the long run—something anyone with experience in chemical management learns fast.
Clear labeling, regular safety training, and a culture where it’s all right to point out a missed glove are practical solutions. It helps when everyone understands what just ten grams of these powders could do if spilled or misused. Consumables like gloves and proper ventilation aren’t frills—they’re insurance policies. All those emergency showers, eyewash stations, and cleanup kits see real action in busy research buildings. Every time a supervisor insists on a safety drill or refresher, I remember old lessons: it’s the things that sneak up on you—like imidazole hydrobromide dust—that can cause the most trouble for people and the environment.
Respect for the chemicals you handle keeps small risks from turning into major headlines. That’s experience speaking, not just text in a safety manual.Purity for any laboratory chemical shapes results. Imidazole hydrobromide works in pharmaceutical research, crystallography, and chemical synthesis. Even a tiny impurity creeps into data, disrupts crystal growth, or triggers messy side reactions. Across the industry, the purity benchmark for imidazole hydrobromide stays at 98% or above. Some suppliers go higher, advertising 99%. These numbers reflect how much of the material is the real compound—while the leftover percent covers residual water, trace metals, or unwanted byproducts.
Skepticism comes easy when reading numbers on a spec sheet. “Greater than 98%” signals most of the bottle holds imidazole hydrobromide, but not all. For pharmaceutical chemists or analytical labs, even that sliver under 2% makes a real difference. A recent study from the European Pharmacopeia flagged that trace contaminants sometimes interact with drug candidates. They can create false results and, worse, confuse safety assessments.
Reliable labs won’t settle for guesswork. Certified manufacturers back up their purity claims with high-performance liquid chromatography (HPLC), nuclear magnetic resonance (NMR) spectroscopy, or mass spectrometry data. Experienced chemists learn to ask for batch-specific certificates of analysis—never just generic product data—and most buyers want a clear view of what tests the supplier ran and how recent that analysis took place.
Over the years, colleagues in medicinal chemistry shared stories of wasted days tracing failed reactions to impure reagents. Usually, the problem didn’t scream out on the label. A slight yellow tinge or a persistent smell tipped them off. For one big molecular library project, our group switched to a higher-purity, fresh lot and overnight the yield on a stubborn coupling reaction jumped from 62% to 93%. That single percent or two gave us cleaner products, less chromatography, and confidence that what we saw in the data came from the molecules, not the bottle’s secrets.
In biological assays, contamination can cause artifacts that skew screening results. Some research has traced cell culture problems back to halide contaminants in supposed high-purity imidazole derivatives. Metals—like iron or copper, even at parts-per-million—act as unwanted catalysts. The smallest slip, if left unchecked, brings downstream trouble no purification step can fix after the fact.
Demand for trustworthy, high-purity chemicals keeps rising. Many companies shift to launching real-time verification technologies. Portable Raman spectrometers have started to appear in some QC labs, offering an extra layer of scrutiny beyond supplier paperwork. That keeps both scientists and manufacturers honest, helping to flag off-spec batches before they hit anyone’s bench.
Researchers weighing imidazole hydrobromide sources should put a premium on fresh, batch-specific documentation. Purity claims mean more when they come with test reports dated close to shipping time. A supplier that responds to technical questions with substance, not brush-off language, wins my trust every time.
In the end, reliable chemicals mean reliable results. The push for higher and more transparent purity standards continues—and it’s well earned by anyone who’s watched real scientific progress stall over a barely detectable impurity.
| Names | |
| Preferred IUPAC name | Imidazolium bromide |
| Other names |
2H-Imidazole hydrobromide Glyoxaline hydrobromide |
| Pronunciation | /ɪˈmɪdəˌzoʊl haɪˈdroʊˌbroʊmaɪd/ |
| Identifiers | |
| CAS Number | 7447-49-4 |
| Beilstein Reference | 26364 |
| ChEBI | CHEBI:63655 |
| ChEMBL | CHEMBL2343281 |
| ChemSpider | 23313 |
| DrugBank | DB11346 |
| ECHA InfoCard | 14d20453-0c3c-4e8d-b1b0-1e3f08b4374b |
| EC Number | 214-675-8 |
| Gmelin Reference | 123379 |
| KEGG | C01726 |
| MeSH | D007093 |
| PubChem CID | 6993474 |
| RTECS number | NR7520000 |
| UNII | VCB22I82U8 |
| CompTox Dashboard (EPA) | DTXSID2039247 |
| Properties | |
| Chemical formula | C3H5N2·HBr |
| Molar mass | 161.02 g/mol |
| Appearance | White to off-white powder |
| Odor | Odorless |
| Density | 1.556 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -0.71 |
| Acidity (pKa) | 5.5 |
| Basicity (pKb) | 4.59 |
| Magnetic susceptibility (χ) | -59.0 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.740 |
| Dipole moment | 3.67 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 93.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -60.1 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed, causes serious eye irritation, causes skin irritation |
| GHS labelling | GHS07, GHS05 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Flash point | 120°C |
| Autoignition temperature | 335 °C |
| LD50 (median dose) | LD50 (median dose): >5 g/kg (rat, oral) |
| PEL (Permissible) | Not established |
| REL (Recommended) | Imidazole Hydrobromide is recommended as a reagent for organic synthesis. |
| Related compounds | |
| Related compounds |
Imidazole Hydrochloride Imidazole Nitrate Imidazole Sulfate Imidazole Acetate 1-Methylimidazole 2-Methylimidazole Benzimidazole Imidazole |