Imidazole didn’t burst onto the chemical scene overnight. The story traces back to the 19th century with Heinrich Debus, who stumbled across it during a reaction involving glyoxal, ammonia, and formaldehyde in 1858. Since then, chemists have refined the synthesis, using routes like the Radziszewski reaction to boost yields and simplicity. The availability of cheap raw materials after World War II pushed imidazole from an academic oddity to a routine building block. Pharmaceutical giants and academic labs alike turned to imidazole rings to construct modern antifungals, beta-lactamase inhibitors, and enzyme mimics. Engineers put it to work in corrosion inhibitors, dye intermediates, and ionic liquids. Watching generations of researchers tinker with this structure, it’s clear the imidazole backbone has driven countless innovations and opened doors far beyond what Debus could ever have imagined.
As a five-membered heterocycle with two nitrogens, imidazole stands out in the laboratory catalog. You will find it sold as slick, colorless crystals or just as often, a white-to-pale powder that carries a faint, almost musty odor. Its approachable melting point, at about 90 to 91°C, makes it easy to handle, melt, or deploy in hot reactions without much fuss. Whether you grab a 99% pure bottle for standard buffers or shop upmarket for pharma-grade lots with precise specs, imidazole forms the backbone for high-performance enzyme purification resins, HPLC modifiers, and specialty reagents. The industry treats imidazole as a staple — priced accessibly, with global production clusters supplying tons for research and manufacturing.
Imidazole packs versatility into a small frame. Its physical properties draw attention: a relatively low melting point (90–91°C), moderate water solubility (450 g/L at 20°C), and a knack for dissolving well in alcohols, chloroform, and ethylene glycol. With a pKa near 7, this ring often stabilizes metal ions or acts as a base in biochemical systems that need mild but effective proton transfer. Chemically, the molecule resists acids and bases alike, but reacts briskly under alkylation, acylation, and nitration conditions. Anybody handling it on the bench knows it doesn’t throw up unpredictable problems — no wild fuming, explosive residues, or volatile unpredictability — making it ideal for labs ranging from undergraduate synthesis to world-class drug development.
Look at the label of any imidazole bottle and you’ll see a focus on purity, moisture content, and heavy metal limits. Buffers and research reagents demand purity above 99%, often with chloride content kept below 0.02%. Producers ship it with standardized documentation: CAS number 288-32-4, formula C3H4N2, molecular weight 68.08 g/mol, and storage advice — dry, cool, and tightly sealed. Labels also spell out shelf life, lot tracking, and analytical methods used for quality control. This level of documentation enables researchers to work confidently, relying on batch-to-batch consistency and traceability from delivery to disposal.
Traditional synthesis relies on the Debus-Radziszewski route, combining glyoxal, ammonia, and formaldehyde in a straightforward one-pot setup. Over the years, tweaks like solvent-free conditions, microwave-assisted heating, and recyclable catalysts have shaved down waste and cut costs. Scale-up isn’t tricky: industrial setups push through larger crude quantities, then purify with recrystallization or distillation. Greener routes appeal to newer firms — less solvent, fewer byproducts. In my experience, switching to these streamlined methods saves time and keeps the workspace more pleasant, since you’re not bogged down by endless extractions or heavy, smelly waste streams.
Imidazole’s reactivity makes it a chemistry workhorse. Its nitrogens act as nucleophiles — ready for ring-opening, condensation, and alkylation. The aromatic ring endures many a treatment from oxidizers, acids, or even rougher conditions in some synthetic adventures. Add a methyl group, you tune its hydrophobicity. Tether a long chain or functional group, and suddenly it's part of a high-affinity ligand for affinity chromatography or a tailored pharmacophore. Lab folks love using it for peptide coupling, as a base in N-alkylation, or to fashion imidazolium salts for ionic liquids and battery research. The range of chemical playgrounds for this molecule seems to keep growing, especially with new catalysts and reagents opening up next-gen transformations.
Over time, chemists have hung a lot of names on imidazole. Textbooks and catalogs often call it glyoxaline, 1,3-diazole, or even imidazolum when discussing ionic salts. Trade names pop up in documentation: Sigma-Aldrich, Fisher, and TCI label it under related synonyms and registration numbers like EC 206-019-2. In specialty polymer and battery markets, you’ll find it hidden under product series linked to solid electrolytes or functionalized resins. Communication between labs often involves sorting through these different synonyms. For students and new staff, keeping a “crosswalk” of trade names prevents repeat orders or, worse, failed reactions from mislabeling.
Working with imidazole in the lab doesn’t demand extreme caution, but you respect it for what it can do. The dust irritates eyes and the respiratory tract — gloves and goggles aren’t optional, even for momentary handling. Spills or direct skin contact can bring on mild redness or itching; in heavier doses, the risk climbs, particularly during large-scale reactions or prolonged use with solvents. Ventilation in the workspace is key — a sweep with a fume hood pays off. Small-scale disposal calls for collecting solutions separately, then neutralizing with acid or base before shipping off as hazardous waste. Production facilities invest in airtight filling stations, routine air monitoring, and staff training to curb any long-term exposure. Industry pushes toward minimizing dust and vapor generation, tightening operational standards without bogging down productivity.
Look around — imidazole turns up everywhere once you know what to look for. Pharmaceutical researchers depend on its ring in antifungals like miconazole, proton pump inhibitors, and enzyme-modifying drugs. Biochemistry labs use imidazole in affinity chromatography, especially to elute his-tagged proteins. Battery engineers tweak imidazole rings into ionic liquids, searching for solvents that shift the boundaries of electrochemical performance. Chemists add it to corrosion inhibitors, epoxy curing agents, and dyes that color fabrics or printer inks. Sewage plants even deploy imidazole-based chelators to mop up heavy metals. The range of uses, across so many industries, shows how an inexpensive ring can punch well above its economic weight.
Every year, labs publish research pushing imidazole into new corners. Current work includes designing imidazole derivatives as anti-cancer leads that bind DNA, or platforms to detect heavy metals with high precision. Electrochemists test new imidazolium salts for safer batteries. Peptide chemists invent cleaner, greener coupling agents that use imidazole’s mild basicity. Open-access journals burst with synthetic tricks — microwave reactions, greener solvents, and immobilized catalysts all serve to push up yields and cut byproducts. Events like Pittcon and ACS meetings feature seminars on high-throughput screening of new imidazole analogues, showing curiosity has not faded since the 1800s.
Imidazole usually doesn’t rank high on the hazard scale, but in larger doses the devil is in the details. Animal studies point to mild to moderate toxicity; rodents exposed to large amounts sometimes display tremors or lethargy, signs that chronic overexposure is not risk-free. Occupational health guidelines set workplace exposure limits and push ergonomics in packaging and handling. For formulated drugs, regulatory agencies press for thorough chronic toxicity testing — knowing that metabolic pathways can sometimes turn the molecule into more reactive intermediates. Environmental groups watch out for excessive releases, since aquatic life sometimes suffers harmful effects if the compound enters waterways in large concentrations. Regulators and toxicologists work closely to plug these knowledge gaps by testing on new models and sharing findings.
Chemistry never sits still. Researchers see imidazole and its derivatives playing bigger roles in green solvent systems, next-generation batteries, and biocompatible coatings. Drug discovery still mines the ring for new leads, with artificial intelligence speeding up the screening of virtual libraries. Materials scientists modify the ring to unlock fire-retardant polymers or smarter corrosion inhibitors. I see the next decade bringing both classics and surprises: the old standbys in protein purification will remain, but high-tech stretches of science and engineering — clean energy, sensors, even food preservatives — seem ready to give the humble imidazole ring yet another lease on innovation.
Imidazole shows up far from the quiet chemistry labs—it’s tucked in medicine cabinets, hidden in cleaning closets, and woven into certain plastics. At first glance it just sounds like another hard-to-pronounce chemical, but walk into a pharmacy and you’re likely to bump into it in antifungal creams. These creams knock out athlete’s foot, yeast infections, and even ringworm. Over the counter or from a doctor, it gets used because it’s tough on fungi and gentle enough for skin.
Fungal infections cause more than itchy feet. They sideline athletes, keep kids home from school, and can even get serious for folks with diabetes or weakened immune systems. I remember a friend in high school missing soccer practice, embarrassed because of stubborn athlete’s foot. Antifungal treatments with compounds like clotrimazole (which is built from imidazole) got him back into the game after two weeks. Without imidazole as a building block for these meds, doctors and pharmacists would have a harder time helping people recover from these nagging infections.
At the industrial level, imidazole takes on new jobs. Chemists use it for making certain plastics, resins, and even dyes. Some companies add it to corrosion inhibitors—those mixtures that help prevent machinery or pipelines from rusting. When hats, jackets or outdoor gear claim they’re mold-resistant, there’s a chance imidazole or one of its cousins played a role in the process. So even if you’re not rubbing cream on a rash, you may still rely on this substance without realizing it.
Doctors and researchers keep coming up with new uses. Some versions have been cooked up to fight parasites or even help treat certain cancers. The thing that stands out is the way scientists lean on the imidazole ring. This five-sided structure links up well with proteins and enzymes found in bacteria, fungi, or even human cells. Because of this, new drugs sometimes start with the imidazole base and get tweaked on the lab bench. One article I read by a pharmaceutical chemist explained how small modifications can lead to totally different effects. Working with a toolkit like this speeds up drug discovery.
The chemistry that makes imidazole useful also means we need to pay attention to safety. Some forms of it can irritate the eyes or skin if handled without care. Like with many synthetic chemicals, too much in soil or water can throw off the balance for plants or aquatic life. Researchers track its presence in wastewater, trying to keep levels down. The push for ‘greener’ chemistry has stirred up talk about safer alternatives and better recycling at chemical plants.
People can use imidazole responsibly by following handling instructions and supporting products developed with less waste and lower toxicity. Chemists have a role to play in inventing biodegradable options or finding natural sources for similar chemicals. Hospitals and clinics can reduce medical waste with better disposal systems for medications. These steps sound simple, but together they help keep the benefits without letting side effects get out of hand.
Imidazole sounds like something out of a chemistry textbook, but it pops up in places you’d never guess: medicines, antifungal creams, and even in some food additives. With information flying in from every direction, it’s easy to start questioning what’s actually going into our bodies. I keep running into the question, “Is imidazole safe for human use?” From conversations with healthcare pros and my own reading, the answer gets a bit nuanced.
This stuff acts as a backbone for several drugs, especially antifungal meds like clotrimazole and ketoconazole. These creams and pills tackle fungal skin problems and infections. Without imidazole, most over-the-counter “jock itch” or athlete’s foot remedies wouldn’t work. The secret here isn’t just in imidazole alone but in the way it’s mixed and the dosages used.
A chemical might sound scary, but so does table salt in the wrong context. Doctors recommend creams and tablets containing imidazole all the time for ringworm or yeast infections. Research shows that, in these forms and amounts, side effects tend to be minor—maybe some skin redness, rarely allergic reactions. Oral versions sometimes mess with the stomach or liver, especially if people take way more than they should or use them long-term.
Food manufacturing sometimes uses imidazole derivatives. You won’t see these in big amounts in your pantry or refrigerator. Authorities such as the FDA keep a pretty close eye on food additives, so substances like this get attention and regular safety reviews. For the average person, the traces that might end up in your meals seem far below levels that would cause harm.
Problems usually show up with misuse or accidental high exposure. If someone works in a factory where they’re handling pure imidazole, breathing it in or getting it on skin could cause irritation. There’s also research linking high doses, mostly in lab animals or in studies far from everyday human life, with some liver problems or other toxic effects. Someone using imidazole creams at home is nowhere near these high-level exposures.
On the flip side, some people want “chemical-free” solutions for everything. From my experience, ditching proven antifungals just because of a scary name can leave minor rashes turning into real medical headaches. No one likes slapping a chemical on their skin, but fungal infections left untreated bring even bigger troubles.
Doctors know imidazole-based medications help clear up stubborn infections. They know the safe ranges. The bigger challenge is keeping people informed. Medicine labels and pharmacy handouts could do a better job explaining that the active ingredients get tested—sometimes more thoroughly than the so-called “natural” alternatives. Educating users, especially about not going off-label or chasing unverified remedies on social media, can cut down on unnecessary fear and mistakes.
People deserve clear, honest info. If you get a prescription or buy a cream over the counter, your pharmacist should answer questions about what’s inside. The science on imidazole may evolve, but sticking with reviewed meds and not exceeding recommended doses keeps things safe. For now, imidazole in its usual forms doesn’t raise a red flag for responsible users. If someone has a known allergy or chronic health issue, then a chat with their doctor matters more than a dive into internet forums. That’s good advice for just about any medicine or ingredient these days.
People hear the word “imidazole” and it doesn't ring many bells. But dig into medicine cabinets and you’ll see it show up in plenty of antifungal creams and pills. Doctors count on it for everyday stuff like athlete’s foot, ringworm, and yeast infections. It’s useful, no question, but side effects don’t get talked about enough with routine medications.
Most people using a cream touch on some mild skin burning, a rash, or irritation. Using it myself on a stubborn bug bite, I remember a tingly, slightly raw feeling for a few minutes right where I put it on. It passed, but for folks with sensitive skin, that can get bothersome quick. Redness, stinging, and itchiness sometimes last all day, making things worse before they get better.
Doctors hand out oral versions for tough infections. That’s where the gut and liver start to join the conversation. Nausea, bloating, a strange metallic taste, or even mild stomach pain might pop up, especially for people who aren’t eating much while they’re sick. Some folks end up with dry mouth or headaches, which can hang around for several days.
Anything passing through the liver needs a closer look, and imidazole falls into that category. My uncle tried a pill version and ended up making four trips to get blood tests. If the body isn’t clearing it fast enough, things get tricky: liver enzymes spike, and jaundice or fatigue hit hard. Cases of true liver injury are rare, but they do happen, especially for people who already have hepatitis or drink a lot of alcohol. The older someone gets, the more sense it makes to ask about their overall health before starting a course.
It’s easy to gloss over allergic reactions. Hives, swelling of the lips or face, or trouble breathing show up unexpectedly and demand an overnight hospital stay or worse. These cases leave a lasting impact—you don’t forget an EpiPen injection or a trip to the ER.
Doctors run checks on drug interactions, but nobody can keep every small detail straight. Imidazole can mess with blood thinners, heart medicines, and some antidepressants by slowing down the body’s ability to break them down. One friend started new meds at the same time as an antifungal. His doctor caught it after he kept feeling dizzy and weak, turning out that drug levels in his bloodstream had doubled. This sort of chain reaction could send someone into a crisis if a pharmacist or doctor isn’t paying attention.
Open conversations with the doctor make the biggest difference. Telling the truth about alcohol use, other medications, and any history with rashes or allergic reactions can change a doctor's decision entirely. It isn’t just about reading the packet insert and hoping for the best. Doctors and pharmacists both should check for hidden risks. For skin issues, starting on a small area helps read the body’s response before diving in.
Most reactions aren’t emergencies, but feeling lousy when you’re trying to kick an infection makes recovery tougher. Quick reporting on side effects leads to better results—nobody should just power through weeks of stomach pain, skin burning, or headaches from a prescription. Trust counts, and so does a real partnership with health care providers.
You notice quickly, working in a lab, how the smallest slip can ruin a week’s effort. Imidazole is one of those chemicals—promising, useful, but tricky if handled wrong. I once watched our reagent cabinet turn chaotic after somebody stacked bottles without much thought. Next morning, the bottle of imidazole showed signs of cake-like clumping, white powder thickened and useless. This isn’t something you just brush off.
Imidazole reacts to moisture in the air. It pulls in water and forms lumps that make it hard to measure accurately—bad news for anyone running a careful experiment. I’ve learned it’s best to keep the container tightly closed. Otherwise, the next project’s numbers drift off, and everyone blames “bad luck” instead of a leaky lid.
Once, someone transferred imidazole into another jar for “convenience.” They used a container from the bench, and days later, crystals turned yellow. That meant either water or air got inside, oxidizing it. What felt like a shortcut wasted a whole supply.
There’s no need for magic tricks here. Store imidazole in a tightly sealed container. Avoid glass jars that don’t close properly—polyethylene or HDPE bottles work well, especially if they snap tight. Every chemical supplier sends the stuff in screw-capped plastic for a reason.
Room temperature works, but keep the jar somewhere dry. Humidity causes trouble fast. Labs with old air conditioning fill quickly with moisture, especially in summer. A dry cabinet or a desiccator makes all the difference. People in busy labs sometimes open the chemical store every few minutes, so a cabinet with a small dish of desiccant adds insurance. It might feel overkill—right up until the day your powder turns to a useless brick.
Lots of folks stash chemicals in the fridge, thinking it keeps them fresh. For imidazole, this usually causes more problems. Cold air leads to condensation inside the bottle—even tiny amounts of water get drawn in. Every time the container goes from cold to room temp, droplets show up inside. After one month on a fridge door, that fine powder picks up moisture and cakes up just as fast.
Good labeling sounds boring, but in my experience, it saves more lab drama than anything else. A clear label with purchase date, opening date, and your initials goes a long way. There can be several jars of imidazole floating around. It helps to know who last opened what and when.
Storing chemicals well saves money, spares people hassle, and keeps everyone safer. No instructor ever emphasized this enough in school. After one too many ruined experiments, though, you start paying attention. It takes just a couple of seconds to close the lid right and stash the jar on a dry shelf. Those seconds give weeks of peace and a better shot at results worth the effort.
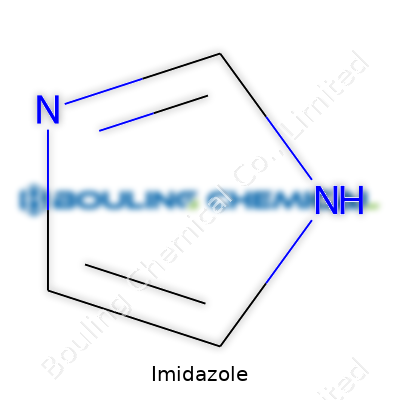
Imidazole stands out in the world of organic chemistry because of its small size and remarkable versatility. The molecule’s ring structure, made of three carbon and two nitrogen atoms, sets it apart from bigger, clumsier structures that often feel less approachable for real-life applications. Picture a pentagon—a five-sided shape—where positions one and three hold nitrogens, with the rest taken up by carbons. This arrangement doesn’t just look good on paper; it gives imidazole the ability to play several roles in both living systems and industrial use.
I’ve come across imidazole most often sketched as a pentagonal ring, with alternating double bonds snaking around. The star players here: two nitrogen atoms, each with its own personality. The first one (at position one in the ring) can share a proton easily, while the other (at position three) bonds tightly to a hydrogen atom. That’s not just trivia—it explains how imidazole manages to participate in so many critical reactions, flipping between acid and base forms with impressive speed.
This structure results in properties that catch attention. Imidazole dissolves well in water and alcohols, which means it ends up in all kinds of places, from pharmaceuticals to biological enzymes. Its electrons are spread just right for stable aromaticity, like you’d see with benzene, but with a more reactive edge courtesy of the nitrogens.
During a summer internship at a pharmaceutical lab, I saw imidazole everywhere—buffer solutions, enzyme stabilizers, even as part of drug scaffolds. The structure allows it to bind metal ions tightly, which becomes crucial for many proteins, especially those involved in metabolism. Without this specific combination of atoms, proteins like hemoglobin might not function as they do. In brewing, biology, and synthetic chemistry, you meet imidazole or its cousins all the time. It’s hard to ignore how a ring so simple at first sight helps anchor life’s most complex machinery.
Imidazole’s basic character means it holds onto protons just enough to help reactions along, but not so tightly that it gets in the way. This balance makes it valuable in buffering solutions, especially where the pH needs stability. Researchers rely on that predictability, whether they’re studying enzymes or coaxing chemical reactions in beakers.
Despite these strengths, imidazole doesn’t escape challenges. Its widespread use can lead to environmental buildup, since many laboratory and industrial wastes contain traces of it. Wastewater treatment needs robust protocols to handle pharmaceuticals and chemical reagents, imidazole included. Green chemistry provides a sensible path forward here, pushing for recycling and safer processing steps. Some labs now focus on developing catalysts that help degrade imidazole-based compounds more efficiently.
On the flip side, the same structure that makes imidazole adaptable also lets researchers design new molecules inspired by it. Medicinal chemists tweak its ring to create antifungals and other medicines. Industrial chemists design corrosion inhibitors and custom catalysts. Progress often comes by making tiny changes to the original imidazole ring, proving that one simple five-membered structure still drives innovation decades after it first caught chemists’ eyes.
| Names | |
| Preferred IUPAC name | 1H-imidazole |
| Other names |
1,3-Diazole Glyoxaline |
| Pronunciation | /ɪˈmɪdəˌzoʊl/ |
| Identifiers | |
| CAS Number | 288-32-4 |
| 3D model (JSmol) | `Imidazole|/p@1.551(1.278,0.000);N@0.866(-1.036,0.000);C@-0.452(-1.148,0.000);N@-1.133(0.082,0.000);C@-0.247(1.183,0.000);H@2.570(1.330,0.000);H@1.384(2.161,0.000);H@-0.975(-2.126,0.000);H@-2.146(0.157,0.000);H@-0.759(2.162,0.000)` |
| Beilstein Reference | 120922 |
| ChEBI | CHEBI:28094 |
| ChEMBL | CHEMBL709 |
| ChemSpider | 50564 |
| DrugBank | DB02007 |
| ECHA InfoCard | 03fa4047-e5d5-4e5c-aa19-cd119198fda2 |
| EC Number | 206-019-2 |
| Gmelin Reference | 8333 |
| KEGG | C00117 |
| MeSH | D007084 |
| PubChem CID | Imidazole's PubChem CID is: "795 |
| RTECS number | NR7170000 |
| UNII | IM4R6JY0EJ |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DTXSID8022552 |
| Properties | |
| Chemical formula | C3H4N2 |
| Molar mass | 68.08 g/mol |
| Appearance | White to pale yellow crystalline powder |
| Odor | ammonia-like |
| Density | 1.03 g/cm³ |
| Solubility in water | miscible |
| log P | 0.02 |
| Vapor pressure | 0.0004 mmHg (25°C) |
| Acidity (pKa) | pKa = 6.99 |
| Basicity (pKb) | 7.0 |
| Magnetic susceptibility (χ) | −7.9×10⁻⁶ |
| Refractive index (nD) | 1.616 |
| Viscosity | 2.6 cP (20 °C) |
| Dipole moment | 3.67 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 146.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | 113.0 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | −1816 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | G01AX05 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes serious eye irritation, causes skin irritation. |
| GHS labelling | GHS02, GHS05, GHS07 |
| Pictograms | GHS07, GHS08 |
| Signal word | Danger |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P280, P304+P340, P312, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | 50 °C |
| Autoignition temperature | 801 °C |
| Explosive limits | Explosive limits: 2-32% (in air) |
| Lethal dose or concentration | LD₅₀ (oral, rat): 970 mg/kg |
| LD50 (median dose) | LD50 (median dose): 970 mg/kg (rat, oral) |
| NIOSH | MV9450000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Imidazole is 0.1 mg/m³ |
| REL (Recommended) | 50 mg/m³ |
| IDLH (Immediate danger) | 800 ppm |
| Related compounds | |
| Related compounds |
2-Methylimidazole 4-Methylimidazole Benzimidazole Histidine Histamine |