Imidazole-1-acetic acid did not pop onto the scientific scene overnight. Its origins stretch back to the early investigations around heterocyclic compounds, especially as researchers hunted for molecules related to histamine metabolism. Pioneers recognized that tweaking the imidazole ring built pathways into a wide range of biological and pharmaceutical discoveries. Labs in Europe and the US both sprinted to publish findings once basic syntheses became viable in the twentieth century. The drive came partly from efforts to understand histamine’s metabolic fate, steering the attention toward derivatives like imidazole-1-acetic acid. Over the past few decades, waves of research grew as detection and analysis tools improved. More than a footnote, this molecule has a story that keeps running in parallel to shifts in biochemical research priorities.
On the shelf, imidazole-1-acetic acid shows up as a crystalline powder or sometimes as small grains, stable under everyday conditions. In the catalogues of chemical suppliers, it gets listed with plenty of alternate names, often depending on the research or industrial context. Its presence shines as an intermediate, an analytical standard, or as a target in bioassays. Those who have ordered it for lab work know that it doesn’t rank among the most expensive reagents, which makes it a reasonable choice when scaling up from exploratory syntheses to more ambitious projects. The compound's moderate solubility fits the hands-on needs of researchers running a wide range of physiological or organic chemistry studies.
Imidazole-1-acetic acid stands out with a molecular formula of C5H6N2O2 and tips the scales at about 126.12 g/mol. Its melting point hovers close to 194°C, putting it well above most standard laboratory room temperatures, making accidental liquefaction a non-issue. Water solubility isn’t exceptional or poor, balancing between trickier hydrophobic reagents and completely aqueous solutions. The compound carries both acidic and basic traits, thanks to the imidazole ring and carboxylic acid group, making pH adjustments a straightforward, predictable task. Researchers working with this molecule can handle it with the expectation that it will not decompose or oxidize rapidly, which sidesteps a fair amount of troubleshooting.
Suppliers typically ship imidazole-1-acetic acid with purity ratings above 98%, with accompanying certificates verifying batch specifics, residual solvents, moisture content, and appearance. Labels note hazards sparingly, based on known data, leaving storage advice to a dry, well-ventilated place, sealed carefully. Orders above the gram scale often come with safety datasheets tucked in, a workplace reminder for both research and teaching labs that documentation must keep pace with chemicals in use. Clarity in technical sheets dimensions clearance for regulatory compliance and safe application in both development and manufacturing settings.
Early routes for synthesizing imidazole-1-acetic acid wrestled with poor yields, often relying on complex organic syntheses or lengthy extraction from biological samples. As lab procedures matured, chemists moved to routes starting from glyoxal and related aldehydes, cyclizing with ammonia and acetic acid in the presence of catalysts. Scaling these reactions turned up practical issues, especially when handling raw materials in large batches or purifying the product without introducing contaminants. These days, the focus leans toward green chemistry, aiming for procedures that drop hazardous byproducts and improve atom economy. Automation in synthesis now lends a steady hand, making product quality less dependent on who stands at the bench.
Modification of imidazole-1-acetic acid delivers a window onto the versatility of the imidazole moiety itself. Its carboxylic acid group reacts well in amidation and esterification, creating new derivatives that serve uses across pharmacology and biochemical research. The imidazole ring supports N-alkylation and halogenation, which researchers exploit to probe enzyme activity and develop potential therapeutic agents. Such reactivity keeps the molecule relevant not only in drug design but in the fine-tuning of probes for analytical methods, giving experimental chemists a toolkit with breadth and depth. Working hands-on with these modifications, frustration occasionally meets delight as unexpected reactivity throws a puzzle into even a well-planned experiment.
Imidazole-1-acetic acid turns up under sticker names like 1H-Imidazole-1-acetic acid, Histaminoic acid, and Imidazoleacetic acid, each labeling reflecting focus on its structure or biological background. Product numbers and CAS registries help cut confusion, which comes as a small mercy in catalog searches or regulatory reports. In my own desktop chemical inventories, tracking synonyms kept more than one project from running aground due to a labeling mismatch between suppliers or journal articles. Consistency in nomenclature isn’t just a paperwork task — it shapes reproducibility and guards against error, especially as samples change hands between labs or during scale-up.
Experience in both education and industry makes clear the value of safety. For imidazole-1-acetic acid, hazard ratings stay low compared to more reactive compounds, but standards require gloves and standard lab attire for handling. Ingestion, eye contact, or inhalation risks match other organic acids. Working in a fume hood meets best practice, particularly during weighing and solution preparation. Disposal in accordance with municipal guidelines keeps compliance on track. Keeping records of exposure and reviewing the material safety data sheet forms the backbone of responsible operation. Training new researchers on these basics builds confidence and prevents costly mistakes or injuries.
Imidazole-1-acetic acid finds work on both sides of the academic-industry divide. Biochemistry uses it as a marker for histamine metabolism, offering clues into inflammation, neurotransmission, and immune function. Drug discovery leverages its structure for analog creation with hopes of new anti-inflammatory or antiallergic agents. Beyond pharmaceuticals, its role as an intermediate in custom synthesis or as a tool for calibration in analytical chemistry fits neatly with the steady grind of research and production labs. Analytical chemists tune instrumentation with known standards, tracing benchmarks set by imidazole derivatives and stretching progress in diagnostics.
Projects tackling metabolic pathways or evaluating gut flora function have turned to imidazole-1-acetic acid as a biomarker. Its connection to neurological and allergic responses continues to attract attention, as labs look for better ways to trace disease onset or monitor therapy. Product development teams in the pharmaceutical sector survey its core for new leads, exploring patents and optimizing analogs based on new molecular insights. The push for more sustainable synthesis also runs through both academic and industrial R&D, laying down challenges in minimization of waste or maximization of product purity. Lessons learned from running reactions at the bench trickle up into better workflows and safer, cheaper processes.
Imidazole-1-acetic acid slips onto toxicological screens with a supporting, not starring, role. Metabolic studies map its fate, flagging safe dosage thresholds that allow its use in animal and cell models with relatively low risk. Acute and chronic exposure research reports limited adverse effects at standard research concentrations. Larger scale toxicity profiles sit on file, not because the chemical triggers urgent alarms, but because regulation and consumer safety demand vigilance. My experience underscores the value in never assuming a molecule’s safety on sparse data — disciplined study and prudent skepticism work better than shortcuts or assumptions.
Imidazole-1-acetic acid stands at an interesting crossroads. Research into metabolic biomarkers builds fresh motivation to refine assays and improve detection methods, both in health sciences and drug development. As interest in histamine pathways grows, so does the crowd of researchers sizing up this compound as a window into new therapies or as a mediator in disease. Technological improvements in synthesis, testing, and data analysis sharpen the edge for both clinical translation and pure science. Environmental impact and greener chemistry raise hopes for cleaner, safer methods. I’ve seen more researchers stepping into collaborations, sharing data and pooling insights, breathing new energy into a molecule whose history started quietly but now pulses with possibility.
Imidazole-1-acetic acid doesn’t often turn up in dinner conversations, but the things it helps us learn reach into our daily experience. This molecule grabs the attention of neuroscientists, pharmacists, and even folks interested in the fine details of metabolism. I've seen how tightknit the relationship between chemicals like this and real changes in the brain can get, especially in academic labs where curiosity guides the whole project.
Imidazole-1-acetic acid draws so much interest because it tells a piece of the histamine story in the body. Histamine gets a lot of fame for allergies and sneezing, but it's also critical in sending signals through the brain. Researchers keep using imidazole-1-acetic acid as a way to look at how histamine gets broken down. Students prepping for exams sometimes forget that nervous system chemicals lead complicated lives—histamine goes from excitement in nerve cells, powers reactions, and eventually winds up as imidazole-1-acetic acid. Measuring this molecule lets scientists see how active histamine has been over time. That’s detective work you don’t often hear about outside of research.
In the years I spent in academic research, pharmaceutical development teams would talk about imidazole-1-acetic acid when comparing how new drugs change brain chemistry. Testing animals or human samples, researchers track levels of this molecule to watch for shifts in histamine activity. This opens up clues about sedation, alertness, or even some features of mental illness. It’s rare to spot toxic changes from this molecule itself, which makes it a useful marker—almost like a time-stamped receipt after a chemical shopping spree.
Imidazole-1-acetic acid sometimes appears in studies about epilepsy, schizophrenia, and sleep disorders. I remember one neurologist mentioning how tracking the changes here can point out where histamine pathways are off track. This has real impact—think about kids with attention issues, or people who can’t shake off grogginess after sleep. If a new medicine starts to push histamine breakdown one way or another, the levels of this molecule might signal whether those changes mean better days or more confusion.
To solve health problems tied to brain chemistry, scientists collect every clue they can. Lifting the lid on how histamine lives and dies in the brain means experts don’t stumble around in the dark. Imidazole-1-acetic acid offers a spotlight. If you know exactly how high or low its level should be, you have a better shot at designing drugs that nudge things back toward normal. Many projects can get stuck if the story ends too soon, but with this chemical as a checkpoint, the work keeps moving forward.
Knowing about molecules like imidazole-1-acetic acid won’t change the morning commute or lunch choices, but it does ripple out. Each data point in the lab brings us a step closer to real breakthroughs: safer allergy medicines, progress in mental health, and sharper insights into how our brains tick. It's a behind-the-scenes actor, never grabbing headlines but quietly keeping research honest and moving.
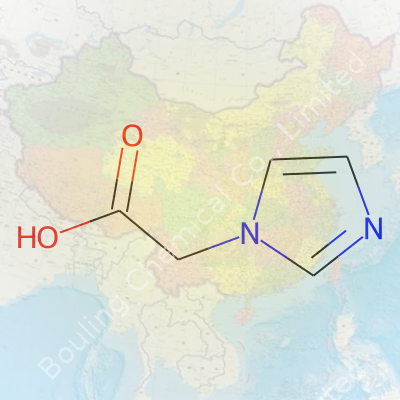
Imidazole-1-acetic acid may look like a mouthful to anyone unfamiliar with organic chemistry, but its makeup actually follows some rules chemists use every day. It starts with the imidazole ring — a five-membered structure with two nitrogen atoms holding things together at the one and three positions. Attaching an acetic acid group to this ring at the “1” spot changes its personality in big ways.
Let’s get specific: the chemical formula for imidazole-1-acetic acid is C5H6N2O2. The structure carries that imidazole base, which you can picture as a pentagon with two nitrogens at non-opposite edges. At the “1” position — right next to one nitrogen — you find the extra piece: a two-carbon chain ending with a carboxylic acid group (–COOH). If you’re used to drawing molecules, start with the pentagon of imidazole, and stick the acetic acid group off the first nitrogen’s site.
Chemists often sketch this as a five-membered aromatic ring, with nitrogens at positions one and three, then a –CH2COOH chain attached to one of those nitrogens. The delocalized electrons on the ring make the molecule fairly stable, but that free carboxylic acid adds another layer of reactivity. These details matter, especially for reactions in the lab or for its role inside living cells.
This compound doesn’t only show up on chemistry tests. It’s a real player in biology. In humans and many animals, it acts as a breakdown product of histamine, one of the main chemicals involved in immune responses and allergies. Enzymes in the liver convert histamine to imidazole-1-acetic acid before removal from the body. Measuring the levels of this molecule in urine or blood can help doctors and researchers understand how histamine is being processed. Too much or too little gives clues about how the body handles inflammation.
Pharmaceutical researchers, always searching for new approaches, look at compounds like imidazole-1-acetic acid as potential templates for drug design. Having both an aromatic ring and an acid group opens doors: it sticks to enzymes or cell receptors and may tweak biological processes. Drugs built on similar imidazole networks have brought treatments for fungal infections, heartburn, and a few nervous system disorders.
With imidazole-1-acetic acid, the issues often come down to how we make and measure it. In the lab, getting a decent yield means controlling for temperature and pH — both impact purity. In clinical settings, tracking the compound accurately in a sea of similar molecules asks a lot of our test equipment. Labs have turned to technologies like high-performance liquid chromatography (HPLC) and mass spectrometry just to keep up.
I’ve seen research teams wrestle with the fine points of imidazole ring substitutions. Getting the right compound, free of contaminants, eats up time and money. Better synthesis techniques would help, as would standardized protocols for clinical labs. If the broader scientific community could agree on a universal test method, everyone from hospitals to pharma would save time and resources.
What seems like a minor chemical can tell a much bigger story about health, disease, and the power of chemistry to drive change. The journey from imidazole-1-acetic acid’s structure to its real-world use covers research, medicine, and even industrial chemistry. Solutions don’t just involve new molecules; they call for better teamwork across science and medicine. The more people recognize the vital role smaller compounds play, the stronger the foundation for future breakthroughs.
Storing chemicals is not just a checklist task for labs and industries. These details can shape safety, product lifespan, and even costs. Imidazole-1-acetic acid stands out because its structure and properties raise specific stakes. Anyone who’s spent time in a chemistry stockroom or worked with delicate compounds learns quickly that smart storage is at least as important as proper measuring or labeling.
It only takes one hurried stowing on a humid afternoon to ruin an entire batch. Imidazole-1-acetic acid pulls in moisture from the air if left out in open containers. A little water can set off unwanted reactions, which makes the material less predictable. I once saw an experiment's data look completely odd because a researcher borrowed a small scoop from a bottle left open just for a couple hours. In our lab, we learned the hard way—tight lids, right away, every time.
Room lighting rarely makes headlines, but prolonged exposure can start subtle changes in many chemicals, including imidazole-1-acetic acid. Fluctuations in temperature—especially in workrooms without stable climate control—speed up those unwanted shifts. I remember frustration during summer months, tracking down weird results, only to realize the back shelf turned into a sauna after maintenance changed the air flow. Keeping this acid in a cool, dry cabinet, away from the sunlight, keeps its character intact.
Plastic bottles offer convenience, but glass often makes a better home for this particular chemical. Glass resists possible reactions with the acid and shrugs off changes in humidity better. Opaque glass helps, giving another layer of protection against stray light. In shared workspaces, clear labeling saves a ton of headaches, especially since many small bottles look nearly identical once powder dusts the outside.
Even with perfect storage, accidents and spills will occasionally pop up. Quick wipes with just any rag won't cut it—safeguards like designated spill kits and proper gloves cut down risk when cleaning up. I once watched an intern skip gloves to mop up a small spill, only to find skin irritation flaring up within hours. Quick response, disposal in clearly marked containers, and immediate washing of surfaces all help prevent long-lasting problems down the road.
In academic labs, turnover stays high as students cycle through in a few semesters. Training should reach beyond just reading a label. Practicing what works, like immediate recapping, careful measuring, double-checking that everything gets sorted into the right shelf space, lowers the odds of mistakes. Nobody wants to be the reason a project stalls because of a preventable contamination.
Smart handling of imidazole-1-acetic acid sounds simple on paper, but real trust in results—and real safety—depend on routines built through both training and experience. From tight lids to glass bottles and cool, dark storage rooms, every small step adds up. In my own work, sticking to the basics not only protected the chemical, but sometimes, it saved the whole experiment from going off track.
Staring at the label 'Imidazole-1-acetic acid', plenty of people feel the usual chemical shivers. Lab techs know the sensation: unfamiliar words can mean hidden dangers. Yet, it pays to look up the facts before treating a compound like a ticking time bomb. Imidazole-1-acetic acid pops up in scientific research and pharmaceutical labs, often as a building block in biochemical studies. Some might say handling any organic compound calls for caution, but I’d rather look at the actual record.
Most safety documents, like Sigma-Aldrich and ChemSpider, agree on one thing: this compound doesn’t leap out as particularly risky. The toxicity data is slim, and nothing screams acute danger. I’ve spent hours chasing down horror stories involving this molecule—came up empty. No cases of serious burns, allergic reactions, or violent exothermic boosts. Comparison matters here. Plenty of bench chemicals demand thick gloves and face shields. Imidazole-1-acetic acid rarely makes that list.
It’s not flammable. The dust doesn’t drift into dangerous clouds. No reactivity with air or water. Even so, I remember a basic rule from years of shared lab space: just because something isn’t a heavy hitter doesn’t mean you treat it like sugar or salt. Standard lab habits—gloves, goggles, lab coat—cut down on the unknowns.
The broader literature describes this molecule as part of normal metabolic pathways in mammals. If the body can handle breakdown products of this compound, it suggests low hazard. Now, a person off the street shouldn’t go inhaling powders or swallowing pills without context, but working scientists won’t find Imidazole-1-acetic acid in the 'danger zone.' No mandatory respirators. No splash hazard warnings. SDS pages, if read all the way through, highlight basic hygiene and good lab practice. Wash hands after use. Don’t eat or drink in the work area.
At home, you likely can't even buy this without signing off with a business or a research credential. Outside organized lab settings, access is limited, so exposure risk for the public sits near zero.
I’ve seen smart researchers treat low-profile chemicals with respect, and I’ve watched careless coworkers cut corners and make messes. Oversight—like proper labeling and a clean bench—beats fear or ignorance. The best solution against rare but possible mix-ups lies in education. New staff and students need to see demonstration, not just words on a paper. Ask for the SDS, look for the basic hazard phrases, and store chemicals in a cool, dry spot away from food or strong acids and bases.
Even scientists occasionally forget the basics. Open container, powder flies. Water bottle nearby. Somebody distracted. Mess happens. Taking a few seconds to read the label and set up your workspace right never feels wasted. Institutional policy should support routine training. The cost of an extra pair of gloves beats the mess—and stress—of sorting out a spill or allergic flare-up.
There’s wisdom in not jumping to conclusions because a substance carries a winding name. Imidazole-1-acetic acid teaches the larger lesson: not every chemical signals disaster. Test the facts, build good habits, and put energy into smart prevention, not anxious overreaction. Chemistry stays safe for everyone if respect and common sense take the lead.
I’ve seen plenty of scientists obsess over reagent bottles, scanning every detail on the label before bringing a single drop into the lab. Imidazole-1-acetic acid is one of those chemicals that prompts a closer look. Nuanced as the name sounds, purity holds real weight in how researchers handle this compound. The grade stamped on the bottle shapes everything—reaction success, cost, safety, and waste. There's nothing like a sticky experiment to make you realize a cheap shortcut can end up costing more time and money.
Imidazole-1-acetic acid pops up in a few key grades across suppliers. For those working in chemical synthesis, the reagent grade sits at the baseline. Reagent-grade usually clocks in above 97% purity, and for most chemical reactions in the lab, this works just fine. You get predictable results, fewer surprises in side reactions, and the consistency lets people compare results across different studies without raising eyebrows.
Once you step into sensitive analytical work or prepping materials for pharmaceuticals, analytical grade takes over. Here, suppliers target 99% or greater purity. The extra step in purification strips away metal ions and organic residue that could trip up high-precision measurements. I’ve watched chromatography runs get ruined by an impurity showing up unexpectedly; that one percent makes all the difference. Waste less time troubleshooting and more time getting valuable data.
Some operations, like those involving advanced materials science or reference standards, demand even higher purity—upward of 99.5% or 99.9%. Only a handful of sources offer this, and the price tag quickly reflects the effort. The difference? Impurities at trace levels could interfere with sensitive instrumentation or fundamentally change behaviors in complex systems. One tiny contaminant hiding out can throw off nanomaterial synthesis or bioassay results, and those issues only pop up after weeks of repeat failures.
You’d think a number tells the whole story, but purity isn’t just a percentage. Different suppliers test for different sets of contaminants. Two bottles marked “98%” could perform worlds apart. For example, traces of other nitrogen compounds, chloride, sodium, or heavy metals don’t always show up on a basic assay. That’s why researchers scramble to read the certificate of analysis or demand third-party testing before trusting a batch with their most expensive project.
Research in biology or medicine adds another twist: less-obvious impurities hurt cell cultures or catalyze unintended reactions. My own group once saw an expensive antibody trial derailed before discovering the culprit was an “acceptable” impurity in a reagent-grade stock. Since then, we’ve leaned heavily on analytical or even HPLC-purified stock for critical work.
So why not always chase the highest grade? Cost towers over most decisions. Go for ultra-high purity, and budgets break fast. A few smart ways out: match the grade to the level of risk, run pilot tests, and check for reliable suppliers with a solid track record. Share notes with peers—or risk duplicating their mistakes.
Day-to-day, balancing price and purity isn’t glamorous but keeps everything moving. Be tough on documentation, question the source, and pay attention to your results before scaling up. Chemicals like imidazole-1-acetic acid don't ruin experiments on purpose; cutting corners on purity usually does.
| Names | |
| Preferred IUPAC name | 2-(1H-imidazol-1-yl)acetic acid |
| Other names |
1H-Imidazole-1-acetic acid Imidazole-1-yl-acetic acid 1-(Carboxymethyl)imidazole |
| Pronunciation | /ɪˌmɪdəˌzoʊl wʌn əˈsiːtɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | 2749-59-9 |
| Beilstein Reference | 136489 |
| ChEBI | CHEBI:18235 |
| ChEMBL | CHEMBL1407 |
| ChemSpider | 10472 |
| DrugBank | DB04241 |
| ECHA InfoCard | 100.021.246 |
| EC Number | 3.5.4.4 |
| Gmelin Reference | Imidazole-1-Acetic Acid: Gmelin Reference 8205 |
| KEGG | C00794 |
| MeSH | D007058 |
| PubChem CID | 83515 |
| RTECS number | NR4020000 |
| UNII | 4B8VZV282P |
| UN number | UN3335 |
| CompTox Dashboard (EPA) | DTXSID0035763 |
| Properties | |
| Chemical formula | C5H6N2O2 |
| Molar mass | 142.15 g/mol |
| Appearance | White to off-white crystalline powder |
| Odor | Odorless |
| Density | 1.238 g/cm³ |
| Solubility in water | Soluble in water |
| log P | 0.31 |
| Vapor pressure | 7.6E-7 mmHg at 25°C |
| Acidity (pKa) | 6.90 |
| Basicity (pKb) | 6.95 |
| Magnetic susceptibility (χ) | -54.5e-6 cm³/mol |
| Refractive index (nD) | 1.568 |
| Dipole moment | 2.64 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 151.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -184.4 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1566 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS07, GHS05 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315: Causes skin irritation. H319: Causes serious eye irritation. H335: May cause respiratory irritation. |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | Flash point: 230°C |
| Lethal dose or concentration | LD50 (rat, oral): 3800 mg/kg |
| LD50 (median dose) | LD50 >5000 mg/kg (rat) |
| NIOSH | NL |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.5 mg/m³ |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
Histamine Imidazoleacetic acid riboside Imidazole-4-acetic acid Histidine Imidazole |