Chemistry never truly stands still, even for old compounds like hydantoin. You can trace the story all the way back to the 19th century, when Adolph Baeyer synthesized hydantoin in 1861 during his research on uric acid degradation. The discovery set off a string of investigations into the compound’s peculiar five-membered ring, rich with two nitrogens and two carbonyl groups. This simple yet versatile molecule soon caught the eye of chemists hungry for ways to modify and build on basic frameworks. Through the decades, hydantoin showed up repeatedly in medicinal chemistry, synthetic organic research, and agrochemicals. Its ring inspired several successful anticonvulsants and paved the way for later advances in stabilizers for polymers and biocides for personal care formulas. The timeline of hydantoin’s rise is a lesson in pushing the value of a molecule far beyond its first appearance, proving that chemistry’s toolbox always has hidden gems.
Hydantoin sits in the middle of a broad family of imidazolidine derivatives, most recognized by its odorless, somewhat bitter-tasting powder appearance. It won’t pop up in ordinary household products by name, but check the back of many shampoos, creams, and plastics, and you’ll see hydantoin-based preservatives and processing aids at work. As a pharmaceutical intermediate, it provides the backbone for drugs like phenytoin, a globally significant antiepileptic. In industrial use, hydantoin derivatives deliver antimicrobial properties to swimming pools and water systems, keep plastics from breaking down in sunlight, and preserve cosmetics. Chemists lean on hydantoin’s ring structure as a foundation for creativity, tweaking side chains for specific uses or fusing new functional groups to reach desired activity.
The compound looks like small, white crystalline granules, only slightly soluble in cold water and more soluble in hot. Its melting point typically lands around 220-222°C, a number that signals robust thermal stability. The presence of two amide groups and a pair of carbonyls makes it behave differently from simpler rings, offering handy hydrogen bonding that proves valuable in both pharmaceutical and polymer chemistry. The structure remains resistant to degradation under mild conditions. Hydantoin’s chemical backbone has enough flexibility to allow for targeted reactions, but it holds strong during many processing steps. Weight remains moderate, and the compound stands up well to the day-to-day rigors of lab work, which is part of its enduring appeal.
Every batch of hydantoin packed for industry has to meet tight specs. Most producers standardize around purity levels close to 99%, with minimal residual solvents, moisture below 0.5%, and low heavy metal content. Particle size often varies depending on the application, but most labs prefer a medium grind that balances solubility with ease of handling. On the shelves, you’ll find hydantoin labeled with its IUPAC name (imidazolidine-2,4-dione), proper hazard warnings, shelf-life markers, and recommended storage instructions—usually cool, dry, and sealed tight from air. Good labeling helps labs avoid confusion with similarly named derivatives, some of which can have very different safety or reactivity profiles.
Classical synthesis involves heating glycine with urea in a simple condensation reaction, a process first described in the late 19th century but refined over generations for yield and efficiency. Large-scale plants pay special attention to stoichiometry, temperatures, and purification steps, often running the reaction in aqueous or alcoholic solution, followed by crystallization. More recently, green chemistry approaches experiment with environmentally friendlier solvents and catalysts, aiming for cleaner profiles and lower energy use. The original method remains reliable, but process chemists constantly look for shorter reaction times, reusable reagents, and scalable filtration, all intended to cut waste and cost. Routine quality checks after synthesis ensure that no urea or glycine traces linger in the finished product.
Few rings invite as much creative manipulation as hydantoin. The methylene bridge between the two nitrogens serves as a ready site for alkylation, which leads to the family of 5-substituted hydantoins widely used in drug manufacture and specialty chemicals. Halogenation delivers valuable disinfectants for pools and cooling towers. Another favored reaction, the ring-opening hydrolysis, produces a useful set of amino acid derivatives. The presence of two adjacent carbonyls also makes it possible to create condensation products for polymer stabilization. Laboratories often seek new methods for making N-alkyl and N-acyl hydantoins, as these modifications drive further activity in pharmaceuticals and agriculture. The synthetic toolbox grows each year with better catalysts and tweaked reaction conditions.
Though ‘hydantoin’ stands as the most recognized name, the compound comes bundled with synonyms, each used in industry and scientific circles. Look for imidazolidine-2,4-dione on technical documents, or find ‘glycolylurea’ in older pharmacological literature. Brand names rarely use the base compound directly, instead reserving them for functionalized derivatives or specific commercial blends. For pharmaceutical contexts, derivatives like phenytoin or nitrofurantoin carry their distinctive branding, but chemists always anchor understanding in that original five-membered ring. Papers, patents, and chemical catalogs keep the synonym lists handy to avoid confusion, and regulators keep close watch to ensure accurate identification.
Good laboratory practice treats hydantoin with respect, even though its toxicity sits relatively low compared to more reactive cousins. Most industrial-grade hydantoin arrives with clear hazard labels: eye and respiratory irritant, potential for skin sensitivity. Spillage requires prompt containment and cleanup with gloves and goggles. Facilities follow established protocols for storage—cool, dry, and away from incompatible chemicals like strong acids or oxidizers. OSHA guidelines highlight the need for ventilation in processes where dust or aerosols may develop. Fire risk remains low, but hydrantoin dust can ignite under unusual circumstances, so manufacturers suggest keeping ignition sources away from bulk storage. Responsible disposal routes hydantoin waste to chemical collection rather than ordinary landfill, protecting water tables and urban environments.
Hydantoin broadly supports the daily grind of several industries. Its derivatives help preserve cosmetics, extend the shelf life of paints, control microbial growth in swimming pools, and serve as intermediates in drug synthesis. In agriculture, hydantoin formulations deliver biocidal and fungicidal power to crop protection strategies. The pharmaceutical sector draws heavily on 5,5-disubstituted hydantoins for seizure medications—phenytoin, ethotoin, and methsuximide, to name a few. Research institutions dig deeper, leveraging the scaffold to build smart materials and explore enzyme mimics. In the polymer space, hydantoin-based stabilizers prevent UV breakdown in plastics and resins, holding off yellowing and brittleness for months or years. Handling and regulatory rules differ by category, but the underlying ring structure keeps showing up, quietly providing value across multiple fields.
Ongoing interest keeps pushing the science of hydantoin forward, as researchers chase new drugs, more effective biocides, and greener synthesis strategies. Biomimetic approaches model enzymes that transform hydantoin under mild conditions, giving industry routes to amino acids and specialty chemicals not easily made by other means. The search for next-generation anticonvulsants keeps medicinal chemists busy tuning potency, side-effect profiles, and metabolic stability. Newer studies focus on hydantoin-linked peptides and polymers as functional materials in sensors, catalysis, and advanced electronics. The research landscape never loses momentum, driven by both market demand and academic curiosity.
Extensive animal and cell-based studies chart the effects of hydantoin and its derivatives across biological systems. Pure hydantoin itself generally rates as low in acute toxicity; oral LD50 values in rodents often exceed several grams per kilogram. Chronic exposure raises separate questions—certain substituted derivatives (some bearing halogen groups) show higher toxicities and possible links to allergies or irritation. In cosmetics, regulatory agencies limit how much hydantoin-based preservative can end up in consumer products, especially given the public’s heightened sensitivity toward formaldehyde-releasing chemicals. Ongoing research monitors for mutagenic and carcinogenic potentials, both in environmental releases and in medical use, but measurable risks appear controllable through clear guidelines and responsible manufacturing practices.
Hydantoin’s journey isn’t finished. As green chemistry sharpens its focus, demand grows for fully bio-based routes to hydantoin, closing the loop on waste and improving environmental outcomes. In medicine, opportunities exist to discover next-generation anticonvulsants, cancer therapeutics, and diagnostic tools based on hydantoin scaffolds. Material scientists design new smart polymers—responsive to light, heat or biochemical triggers—using hydantoin’s reactive core. Progress relies on a mix of innovative thinking and respect for the lessons learned from decades of safe industrial use. With each step, researchers weigh the benefits of new chemistry against the persistent challenge of managing toxicity, cost, and global scale, ensuring that hydantoin’s story stretches further into the century without losing sight of operational safety and societal value.
In my days working alongside chemists, one compound kept popping up: hydantoin. People outside the lab rarely talk about it, yet it sneaks into all sorts of products we use without thinking twice. Let’s break down why so many companies keep turning to hydantoin and what that means for folks like you and me.
The story of hydantoin goes back to the 19th century, but its real spotlight moment came thanks to its role in anticonvulsant drugs. For decades, doctors have trusted phenytoin — a hydantoin derivative — for managing epileptic seizures. When you visit a neurologist, and they mention Dilantin, they’re talking about this very compound. It stabilizes electrical activity in the brain, reducing the chance of seizures for millions of patients globally. People living with epilepsy rely on these treatments, making hydantoin not a chemistry niche, but a pillar in daily medical care.
Walk through a grocery aisle or a pharmacy, and you’ll find hydantoin derivatives at work in personal care items. Take shampoos, lotions, and makeup — you’ll see words like DMDM hydantoin on the back label. Manufacturers add these for one big reason: they help stop bacteria and fungi from growing in the bottle. If you’ve ever opened an old bottle of shampoo and smelled something off, that’s probably a lack of proper preservatives. A tiny bit of hydantoin-based compound can keep products safer and give them longer shelf lives.
Now, not every story about hydantoin is a rosy one. The same compounds that guard against spoilage in cosmetics have drawn scrutiny from health advocates. DMDM hydantoin, for example, releases small amounts of formaldehyde, which alarms folks worried about allergens and other health effects. Some studies link long-term exposure to skin irritation or potential cancer risks, though standard concentrations in cosmetics meet regulatory guidelines in the US and Europe.
People have pushed for more transparency in ingredient lists, and some brands responded by dropping certain preservatives altogether. Speaking from experience, reading tiny ingredient lists quickly gets overwhelming, and it’s not always clear what’s truly safe. This confusion only grows when companies hide behind obscure chemical names.
Interest in green beauty and eco-friendly home products now drives some companies to re-examine old formulations. I’ve met formulators who pour hours into finding plant-based alternatives to synthetic hydantoins. These alternatives claim to do the same job — keeping products safe from microbes — without triggering the same concerns about formaldehyde release. New preservation systems often cost more, and some don’t last as long once a bottle is opened. This can frustrate shoppers looking for both safety and value.
Companies and regulators will keep debating balance: product safety, cost, and consumer health. Hydantoin’s reach stretches from the pharmacy to the bathroom cabinet, proving that one molecule can influence everything from a daily pill to shampoo lather. Staying informed and checking labels is more important than ever, because a simple chemistry lesson can make a big difference in our everyday choices.
Walk down any drugstore aisle and you’ll bump into dozens of skin creams, cleansers, and makeup items that list ingredients most of us struggle to pronounce. For a lot of folks, “hydantoin” pops up under names like DMDM hydantoin or imidazolidinyl urea. These compounds work as preservatives—they stop products from turning into petri dishes, extending shelf life and saving us from tossing out half-used bottles.
From a science perspective, hydantoin compounds release small amounts of formaldehyde over time. This formaldehyde slows down bacterial growth, protecting both the formula and anyone using it. It definitely solves a problem: the moment water ends up in a cosmetic, it becomes a playground for microbes. Without preservatives, even those “organic” creams can go south fast.
That formaldehyde piece draws plenty of concern. We all know breathing formaldehyde vapors over years—say, in a factory—can cause serious health problems. Nobody wants to take risks with their skin, especially on delicate faces and for kids.
Over the years, batch after batch of products has used hydantoin-based preservatives. The American Contact Dermatitis Society tracks ingredients linked to allergic reactions. DMDM hydantoin and its cousins do show up as triggers for a tiny group of people, mostly those with sensitive skin or a formaldehyde allergy. Think red, itchy rashes and the sort of discomfort folks want to avoid.
Most people don’t react to these ingredients, and the levels used in cosmetics are tightly regulated. In the U.S., the FDA and personal care regulations let manufacturers use hydantoin in limited concentrations. Places like the European Union reviewed safety studies, then set rules capping the amount allowed in what touches the skin.
Dermatologists who see patients every day tend to point out that a patch test can single out the few who do react. For those with a known sensitivity, it just makes sense to use products labeled preservative-free, or to scan ingredient lists with a careful eye.
Concerns about cancer risk and hormone changes got attention, mostly because of formaldehyde’s reputation in industrial use. So far, there isn't convincing evidence that the amounts released from skin care products with hydantoin build up or do harm at normal use levels. Bodies like the Cosmetic Ingredient Review Panel and the European Commission have dug through health data and continue to watch for new research.
From a practical angle, the challenge lies in balancing safe products with real-world use. Without preservatives like hydantoin, store shelves would offer far fewer choices, and safety recalls would skyrocket. Going entirely preservative-free often leads to spoilage, which introduces its own health risks.
For those worried about sensitive skin or who want to avoid formaldehyde-releasing preservatives, brands have started switching to alternatives. Look for products preserved with mild acids (like sorbic acid), phenoxyethanol, or natural plant-based options. These aren't perfect either—every preservative can trigger irritation for someone—but the beauty industry keeps evolving formulas as customers ask for more transparency and fewer allergy triggers.
By reading labels and knowing personal skin limits, most people can find safe, effective skin care. If there’s a history of allergies or a rash flared up after trying something new, talking to a dermatologist helps narrow down triggers and find products that fit individual needs.
Hydantoin drugs, with phenytoin being the best-known, help people control seizures. As someone who has seen friends and family manage epilepsy, I know how these medications can change lives. They can let a young person return to school or help a truck driver keep a job. But the same medications bring along a set of side effects that deserve honest attention. If you or a loved one starts on hydantoin, it makes a real difference to understand what could happen—both expected and rare.
Gum problems show up early with hydantoin. Swollen, overgrown gums can make eating uncomfortable and even change someone's smile. Dentists see this so often that they ask about seizure medications as soon as they see this pattern. Fatigue and sleepiness touch a lot of users as well. Tasks that once felt routine—reading a book, driving, handling tools or heavy machinery—suddenly require more effort or feel downright unsafe. I’ve seen students who once breezed through classes struggle to focus after starting on phenytoin, which points to how the medication slows mental processing for some people.
Hydantoin can also cause tremors, poor coordination, and slurred speech. These downsides often become more visible with higher doses. People sometimes walk as if they’re dizzy, and teachers might notice children tripping over words or losing their balance. Skin rashes pop up, too, and while most rashes stay mild and itchy, some turn into serious allergic reactions—with red, peeling skin or fever that signals the need for emergency help.
Bone health takes a hit with long-term use. One relative developed weak bones after three years on phenytoin—something her doctor connected to trouble with vitamin D and calcium metabolism. Studies show that hydantoin speeds up the breakdown of vitamin D, which then lowers calcium. People face a greater risk for fractures, even with minor falls. Regular bone checks and supplements need a place in any long-term treatment plan, especially for kids and older adults.
Doctors sometimes see problems with blood cell counts. That can mean bruising more easily, infections cropping up, or unexplained fevers. Most people never see these problems, but routine blood tests give early warning. Liver trouble, another rare but real risk, shows up as yellow skin or eyes, dark urine, or nausea. Regular doctor visits—telling your care team about any new symptoms—helps head off these complications.
People with epilepsy already have a lot to balance. Adding side effects to the daily load can push some to stop treatment. Open conversations with doctors make a big difference. If you brush your teeth religiously and schedule regular dentist visits, gum issues can stay minor. Specific vitamins and bone scans limit long-term harm to skeletons. If a rash or soreness pops up, switching to a different medication early can prevent bigger problems.
Staying seizure-free matters, but so does feeling well. Hydantoin has helped millions, yet its side effects call for attention and real-life planning. Anyone taking this medication should push for ongoing support and regular check-ins to keep surprises at bay. Educating families, teachers, and employers pays off in fewer misunderstandings, fewer missed warning signs, and a better shot at a stable, healthy life.
Many people hear names like hydantoin and phenytoin, and — especially in a pharmacy or hospital setting — confusion pops up often. These terms sound alike, but they don’t refer to the same thing. Hydantoin refers to a simple chemical structure, a starting point for several medications. Phenytoin, by contrast, is a specific drug. The difference isn’t a small detail. For doctors, pharmacists and patients, mixing them up can lead to mistakes in treatment or understanding what a drug does inside the body.
Hydantoin itself doesn’t act as a medication. It’s a scaffold used in medicinal chemistry — scientists build on it to develop different drugs. In practice, hydantoin serves as a building block, sort of like the basic frame of a house.
Phenytoin, on the other hand, grabs the spotlight in neurology and pharmacy. Approved decades ago, phenytoin treats epilepsy by calming down electrical activity in the brain. The FDA first greenlit it in 1953, and it’s still on the market today. For many with epilepsy, it’s a lifeline, especially for certain seizure types. The molecule contains a hydantoin ring but also packs extra chemical groups that change how it works and make it effective for seizures. So, phenytoin is a hydantoin derivative, but not all hydantoins work as antiepileptic drugs. This matters because the risks, side effects, and benefits depend on those extra chemical tweaks.
People managing epilepsy often juggle multiple medications. Mistaking hydantoin for phenytoin, or confusing one for the other, could create dangerous gaps in care. Pharmacists see mix-ups in names cause real world harm. Just as acetaminophen and acetazolamide sound similar but treat entirely different problems, hydantoin and phenytoin aren’t interchangeable.
Dosage, monitoring, and side effect profiles differ hugely. Phenytoin sometimes triggers gum issues, balance problems, and — at high levels — can even cause toxicity that demands emergency intervention. Hydantoin by itself isn’t given as a drug; it just sits in textbooks and chemistry labs. Accurate health information empowers patients to ask questions and double-check their medication bottles, lowering the chance of a medication error.
A clear understanding of names and structures doesn’t just help clinicians. It’s vital for patients who want control over their health journey. Misunderstandings about these names can stretch into online forums, leading to speculation about drug actions and safety. Misinformation multiplies quickly, and trust in medical advice suffers.
Patients can ask their healthcare providers to explain their medication’s role and chemical makeup without hesitation. Professional organizations like the Epilepsy Foundation provide resources for deeper dives into common seizure medications, including those rooted in the hydantoin structure. Public health campaigns can fill in knowledge gaps too, especially in communities with less access to pharmacists or up-to-date health libraries.
Errors drop when the conversation between science, medicine, and the person taking the pill gets clearer. Phenytoin and hydantoin sound similar but play different roles. A little curiosity, backed up with facts and understandable language, opens the door to safer care and more personal control over health decisions.
Hydantoin pops up in many places—cosmetics, skin creams, shampoos, even some medications. The chemical structure lets manufacturers preserve products by preventing bacteria and mold from growing in the bottle. It often goes by names like DMDM hydantoin or imidazolidinyl urea on ingredient lists. This shouldn’t surprise anyone familiar with the challenge chemists face in keeping skin creams shelf-stable, especially those meant to last for months in a humid bathroom.
Years back, I struggled with constant itching after using a new face cream. I didn’t suspect the preservative until a dermatologist flagged DMDM hydantoin in the list. The reaction wasn’t immediate but built over days—redness along my jawline, tightening skin, and finally, small bumps that wouldn’t settle down. Cutting out the product stopped the symptoms cold.
Dermatologists confirm my story isn’t rare. Hydantoin belongs to a group of “formaldehyde-releasing preservatives.” These are chemicals that slowly leak small amounts of formaldehyde into products to kill microbes. For most, the levels are low and don’t cause problems. But for a segment of people, this triggers allergies—especially those with a history of skin conditions like eczema or dermatitis. The American Academy of Dermatology points out that formaldehyde allergies tend to sneak up, showing up after repeated use rather than instantly.
Patch tests offer clear answers. Studies from the North American Contact Dermatitis Group show that people allergic to formaldehyde have a much higher risk of reacting to products with hydantoin. A 2020 review in “Contact Dermatitis” highlighted that DMDM hydantoin could cause positive reactions in up to 3% of patients tested for contact allergies. While this might seem like a small slice of the population, in real-world numbers, that’s thousands of people every year dealing with burning, itching, or hives from shampoos or lotions.
Products labeled as “hypoallergenic” or sold for sensitive skin aren’t always free from these ingredients. Reading small print becomes a habit. Sometimes hydantoin-related preservatives lurk under alternate names, which makes spotting potential allergens on ingredient lists challenging.
Being aware of what goes on the skin brings peace of mind. Finding companies that list full ingredients, transparency about formulations, and clear communication from manufacturers really helps. A good rule: patch test any new product on a small part of your skin, even if you’ve never had a reaction before. For those with known sensitivities, it pays to look for “formaldehyde-free” or contact a dermatologist for a printed list of preservatives to avoid.
Product makers have started responding by offering preservative-free options, or by using less allergenic alternatives. Some have moved to potassium sorbate, phenoxyethanol, or others, though new preservatives come with their own caveats.
No one deserves to feel anxious about slathering on a face cream or using body wash. If something doesn’t sit right, trusting your skin’s signals helps prevent long-term damage. Listening to those signals, sharing honest experiences, and supporting stronger labeling standards go a long way in protecting everyone’s skin health.
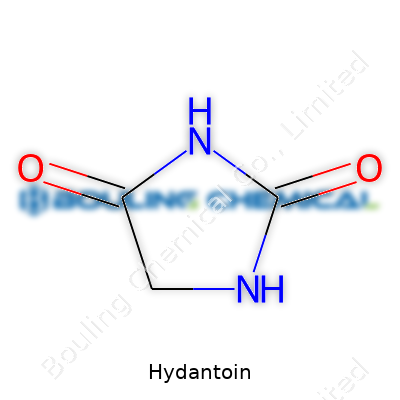
| Names | |
| Preferred IUPAC name | imidazolidine-2,4-dione |
| Other names |
Imidazolidine-2,4-dione Glycolylurea |
| Pronunciation | /haɪˈdæntɔɪn/ |
| Identifiers | |
| CAS Number | 302-02-5 |
| 3D model (JSmol) | `3D Model (JSmol) string for Hydantoin:` `3DSTRUCTURES: 1H,3H-imidazole-2,4-dione` `C1(=O)NC(=O)N1` |
| Beilstein Reference | Beilstein Reference: 80660 |
| ChEBI | CHEBI:37583 |
| ChEMBL | CHEMBL1422 |
| ChemSpider | 6002 |
| DrugBank | DB00949 |
| ECHA InfoCard | 03bb7f2b-e40c-4be4-8889-491fffe0c8be |
| EC Number | 3.5.2.2 |
| Gmelin Reference | 4333 |
| KEGG | C00210 |
| MeSH | D006826 |
| PubChem CID | 789 |
| RTECS number | MU7175000 |
| UNII | 25X51I8RD4 |
| UN number | UN2811 |
| Properties | |
| Chemical formula | C3H4N2O2 |
| Molar mass | 100.07 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.42 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | -0.47 |
| Vapor pressure | 0.000045 mmHg at 25°C |
| Acidity (pKa) | 8.3 |
| Basicity (pKb) | 11.76 |
| Magnetic susceptibility (χ) | -60.7e-6 cm³/mol |
| Refractive index (nD) | 1.597 |
| Dipole moment | 4.50 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 159.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -541.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1529 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | N03AA01 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Danger |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 177°C |
| Autoignition temperature | 194°C |
| Lethal dose or concentration | LD50 (oral, rat): 3,150 mg/kg |
| LD50 (median dose) | LD50 (median dose) of Hydantoin: "500 mg/kg (oral, rat) |
| NIOSH | HY1225000 |
| PEL (Permissible) | PEL: 5 mg/m3 |
| REL (Recommended) | 0.02 mg/m³ |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Imidazolidinedione Allantoin Dantoin Phenytoin Thiohydantoin |