Homopiperazine has stories going back to the mid-20th century, when chemists started playing with new ways to loop nitrogen atoms together. It took shape as scientists explored ring structures similar to piperazine, searching for compounds with potential utility in synthesis and pharmaceuticals. Its simplicity made it appealing—a six-membered ring with two nitrogen atoms spaced apart, not side by side. This small tweak sparked curiosity across labs in Europe and the US. The compound drew attention for its ease of modification and new possibilities: early interest circled around its use both as a building block and a tool for making more complex molecules. Patents and technical papers from those decades show people poking at this molecule, testing boundaries, striving to discover just how useful such a foundational chemical could be.
Look at homopiperazine today—chemistry supply houses stock it in solid form, selling to research labs and innovators alike. The compound works as a core part in creating active ingredients for drugs, complex ligands for metal-catalyzed reactions, and specialty polymers. You’ll find its fingerprints on the blueprints of custom materials and next-generation medicines. Its structure makes it a favorite for those who need predictable behavior without unnecessary fuss, and the clear demand lines up among chemists chasing efficiency as well as flexibility in their synthesis strategies.
Homopiperazine typically appears as a white crystalline powder, soluble in water and many organic solvents. The molecular formula lands at C5H12N2, and the melting point during laboratory handling sits in the 39–41°C range. A two-nitrogen ring gives it a basic character, leading to a sweet spot for chemical reactivity and salt formation. The molecule reacts easily under both acidic and basic conditions, which means it can play several roles—from acting as a base, coordinating to metals, or providing a platform for further chemical additions. Chemists often talk about its reliability—consistent reactivity, and good stability under shelf storage.
Producers outline specifics like assay percentage (usually above 98%), low moisture content, and minimal presence of related impurities. Visual checks show it free of color or clumping. The standard 25-kilogram drum stands tough against punctures and leaks, and labeling includes hazard pictograms, CAS number (505-66-8), batch numbers for traceability, and recommendations for protective gear. Shipping labs pack Material Safety Data Sheets detailing accident response, flammability, and advice on storing away from acids or oxidizers.
Making homopiperazine on scale usually starts with 1,4-dichlorobutane and ammonia. Controlled reaction between these raw materials forms the cyclic structure. Operators monitor temperature, manage pH swings, scrub excess ammonia, and isolate the product by distillation or recrystallization. Years of tweaking this process have led to fewer byproducts and a better safety profile, thanks to closed reactors and stricter controls around venting gases. Smaller-scale labs sometimes target synthesis with variations using different solvents or starting with different chlorinated intermediates.
One beauty of homopiperazine comes from how it allows chemists to attach groups at either nitrogen atom or modify the ring directly. Alkylation with methyl or ethyl groups creates building blocks for medicines aimed at the central nervous system. Acylation launches new amide linkages, useful in polymer synthesis and drug development. Its cyclic structure opens up possibilities around forming chelates with metals, aiding catalysis or targeted delivery inside biological systems. In the right hands, this core structure unfurls a host of modifications, each step pushing into new territory—whether chasing improved activity or better physical properties.
Ask for homopiperazine, and you might hear it called 1,4-diazacycloheptane, azepane-1,4-diamine, or perhydro-1,4-diazepine. In technical circles, these names help avoid confusion with its more common cousin piperazine. If you’ve worked in R&D or fine chemicals, the label "homopiperazine" stands out, guaranteeing you’re dealing with the seven-membered ring and not any lookalike. Manufacturers stick to these names for compliance and clarity, keeping communications clear from sales to safety reports.
Daily work with homopiperazine pushes teams to follow protocols for handling solids that absorb moisture and dust easily. Contact with skin can lead to irritation, and routine exposure brings risk of headaches or breathing trouble if tanks leak. Regulations frame personal protective gear as standard: gloves, goggles, lab coats, and, for bulk quantities, masks or respirators. Spills cleaned with inert absorbents keep the risk down, and clear emergency plans help minimize issues from exposure or fire. If you’re handling tons per year, proper ventilation, storage in airtight containers, and regular staff training keep trouble at bay and chemists confident.
Chemists making anti-psychotic drugs, researchers building new polymers, and materials scientists hunting for better ion-exchange membranes all reach for homopiperazine. It works in the background during creation of fungicides, veterinary medicines, and stabilizers for resins. Medicinal chemistry groups enjoy the freedom to use its structure as a template for innovation around blood–brain barrier penetration or increasing oral availability. Some emerging uses pop up in custom ligands for catalysis or advanced sensors. The world of semiconductors even sees small roles for this compound, as the electronics industry dives deeper into organic materials needing tight control over nitrogen content.
From my experience digging through patents and new journal articles, it stands out that R&D teams rely on homopiperazine for more than just its synthetic utility. They bank on it for rapid iteration and scaffold-hopping—two important trends in drug discovery. As automated synthesis grows, high-throughput screens now include homopiperazine-based libraries, allowing scientists to test thousands of compounds at once. In the race to develop green chemistry, researchers investigate routes for using renewable feedstocks or milder conditions, aiming for less waste and improved safety. Collaboration drives this work, pulling together synthetic chemists, process engineers, and application scientists to make progress against regulatory, environmental, and technical hurdles.
Laboratories and regulatory bodies run toxicity screens on homopiperazine, using animal models and in vitro cell assays. Acute exposure studies show moderate toxicity: enough to warrant careful handling, especially in bulk processes. Inhalation and skin contact raise higher concerns than small-scale test-tube work, sparking rules around fume hoods, gloves, and restricted access. Long-term studies target both environmental and occupational exposures, checking for links to chronic issues or bioaccumulation. Updates come with new research: some studies suggest metabolites formed during breakdown could interact with biological targets in unforeseen ways. These risks push industry and academia to stay alert and constantly recheck protocols in light of new findings.
Homopiperazine may look simple, but its potential keeps expanding. Next-generation drug discovery, energy storage applications, and specialty polymers keep it relevant in today’s labs. Pushes for safer, greener synthesis and tighter regulatory oversight will probably boost demand for high-purity versions and new derivatives. As artificial intelligence and machine learning help identify underexplored chemical spaces, homopiperazine’s reactivity and versatility look set for a new round of breakthroughs—especially as creative teams figure out how to connect its traditional strengths with urgent needs across medicine and industry. With deep roots and hungry minds driving new research, homopiperazine won’t fade from the chemist’s toolkit anytime soon.
Homopiperazine barely makes the news. You don’t hear kids talking about it in chemistry class, and I’ve never bumped into anyone at a dinner party who wanted to discuss it. Still, if you step into the world of drug development or lab work, talk shifts quickly. Homopiperazine doesn’t look like much, but it pulls some real weight behind the scenes, especially for people who research or manufacture medicines.
Back in college, I remember a professor pulling a bottle from the chemical supply room and telling us, “This one helps stitch molecules together.” Homopiperazine acts like a connector in the pharmaceutical world. Drug chemists use it as a “building block” when putting together new compounds, especially those aimed at treating neurological conditions. Its ring structure makes it a favorite for tweaking new molecules that target diseases or even improve old drugs.
Pharmaceutical companies look to homopiperazine for designing substances that might work as antipsychotics, antihistamines, or even some drugs that deal with allergies or infections. Its presence helps shape the backbone of these medicines, sometimes turning a clumsy, ineffective compound into something that works in the body.
Homopiperazine doesn’t just stick around in the medicine world. If you spend any time in big chemical labs, especially those working on specialty coatings or plastic modifications, you might spot it on supply shelves. Researchers use it to make other chemicals, speeding up reactions or helping molecules bond in the right spot.
Out of personal curiosity, I once checked out a report from a materials science conference, and homopiperazine came up there, too. Turns out, the chemical can help make plastics with extra flexibility or resilience. That’s not something you’d notice from the barcode on your shampoo bottle, but it matters for people designing safer, longer-lasting products.
Working around homopiperazine calls for respect. I’ve met more than one chemist with stories about mishandling things in the lab, and this compound isn’t known for being gentle if used without the right precautions. It can be irritating to skin and eyes, so labs put it behind locked cabinets and demand gloves and eye gear. Bringing attention to safe handling keeps new lab workers from learning the hard way, and it’s one of those chemicals you should keep away from anything outside the lab.
Lots of folks never hear the name homopiperazine, but the world would look different without it. Walk into any hospital and you’re likely closer to something made with help from this chemical. It doesn’t get the spotlight, but it makes things tick a little smoother behind the scenes. Drug research hits fewer dead ends. New possibilities for plastic show up every year.
Bringing more transparency to how chemistry drives medicine and industry should be a goal for all of us. Keeping lab workers trained, and holding companies accountable for safety, helps avoid accidents. If you ever meet somebody who designs drugs or new materials, homopiperazine probably played a small, important role in their work. Sometimes that’s all it takes to change a life or keep a product safe in your hands.
Some molecules play a quiet role in chemistry labs, helping make drugs and materials, but rarely appearing in the spotlight. Homopiperazine is one of them. It looks unremarkable to the naked eye, just another white powder when it's pure. Yet, get down to its structure, and things start to get interesting.
Homopiperazine tells a small story if you look at its name. The ‘piperazine’ part nods to its six-membered ring with two nitrogen atoms. Homopiperazine takes that basic shape and expands it. Instead of a six-membered ring, you get a seven-membered ring, placing the chemical into the azepane family. The chemical formula sums it up as C5H14N2—five carbons, fourteen hydrogens, and two nitrogens.
Picture the structure as a ring. Those two nitrogen atoms aren’t right next to each other; one sits at position 1, the other at position 4, giving us the official mouthful of a name: 1,4-diazepane. That slight separation might not sound like much to a non-chemist, but it changes the chemical’s behavior compared to piperazine or morpholine, its more famous cousins.
Chemists value homopiperazine for the flexibility built into that seven-membered ring. It's less stressed, less tightly wound than smaller rings—making it more comfortable in a wide range of reactions. The presence of two nitrogens means it can act as a building block for lots of drug molecules. The difference between six and seven members in a ring doesn’t seem like a big leap, but on the molecular scale, it opens up whole new possibilities.
Pharmaceutical companies keep an eye on these sorts of molecules. They’re useful not just because they react easily, but because their structure allows for tweaks. Chemists can swap in or attach all sorts of side groups, adjusting the behavior of the final drug. This flexibility paves the way for finding better treatments, sometimes with fewer side effects or improved stability in the body.
Mixing up piperazine and homopiperazine is easy. They sound the same and both involve rings with nitrogens. For the beginners, a look at the skeletal formula can clear things up: homopiperazine’s has an extra carbon, which changes its shape and properties. Serious chemists memorize these differences, but many students slip up during exams.
Lab safety also comes into play. The word ‘homopiperazine’ may never make headlines, but like many amines, it can be irritating. Students, especially, need to respect it: gloves, goggles, and fume hood work are standard. This reminder gets personal for me because I’ve seen carelessness with small molecules lead to hand rashes and headaches—not life-threatening, but far from pleasant.
Discovery in the chemical world often begins with tinkering around with building blocks like homopiperazine. Better tools for visualizing and predicting what these scaffolds can do would make a difference. Cheaper, more reliable analytical techniques help researchers check purity and track reactions more easily. Even in a world of smart machines and artificial intelligence, clear understanding of chemical structure—the kind students gain by drawing out these rings—remains at the core of progress.
For most people, the name ‘homopiperazine’ won’t come up outside textbooks and chemical catalogues. Its quiet presence leads to more effective pharmaceuticals, better research tools, and eventually, advances that trickle down into daily life. Chemical formula C5H14N2 may seem like a string of letters and numbers, but it proves once again that even overlooked molecules matter.
Homopiperazine isn’t a chemical you find in everyday conversations. It pops up in specialty catalogs and advanced synthesis work, more at home in research labs than high school classrooms. I remember years ago, during a graduate project, turning a catalog page and spotting it by chance—one of those curious moments where you realize that science relies on countless hidden players.
Labs in academic and pharmaceutical settings rarely face obstacles getting their hands on homopiperazine. A quick scan through companies like Sigma-Aldrich or TCI will display different grades, packaging sizes, and even a list of shipping restrictions. Navigating those options isn’t difficult for an established lab but can trip up independent researchers or smaller operations. Suppliers respond to clear, documented need. For industry, manufacturers often broker deals for bulk, locking in prices to keep pilot plants running.
Buying lab chemicals isn’t as simple as picking up groceries. The paperwork behind homopiperazine reflects this. Companies carry out background checks to avoid supply falling into the wrong hands. In some countries, simply ordering it as an individual rings alarm bells, requiring buyers to prove their institutional affiliations. Restrictions grew tighter after several incidents of lab chemicals showing up in DIY contexts online. Plenty of legitimate researchers feel frustrated by this, especially early-career scientists still building credibility.
Chemists use homopiperazine for synthesizing new compounds, often for drug discovery. The molecule’s structure makes it useful for building blocks in medicinal chemistry. Some folks working with polymers or specialty plastics use it too. It’s always fascinating to see how a niche chemical, almost invisible to the public, finds itself essential to cancer drug research or material science breakthroughs.
With access comes the burden of safe handling. Most labs strictly enforce protocols — goggles, gloves, fume hoods — partly because regulations demand it but mainly because people remember accidents. I recall a close call with a less-regulated amine compound years ago, which left a colleague sick for a week. Nobody sets out to misuse a chemical, but one careless step can derail a project or worse. Homopiperazine isn’t among the most hazardous, but it’s one more reason for thorough training and up-to-date material safety sheets.
If the scientific community wants to ensure steady access, suppliers and buyers both can update their processes. Clear documentation and traceability already help. Investing in new tracking technology, digital inventories, and real-time shipment status reduces delays. Training programs for buyers new to procurement could walk through each stage, so frustration doesn’t build up. Changes like these may sound bureaucratic, but they cut down on confusion and keep the chemical flowing where it’s needed.
Chemistry never stands still. Labs reach for strange, hard-to-pronounce tools like homopiperazine every day, each vial a step toward new cures and inventions. Sometimes all a scientist really wants is a straightforward way to get supplies and a fair shot at discovery.
Anyone with experience in a laboratory or chemical warehouse recognizes the risks that come from ignoring common sense. Homopiperazine isn’t a chemical that generates headlines, but the hazards of poor storage tend to make their own news—with fires, spills, or ruined shipments.
People sometimes underestimate basic risks in the chemical world. Homopiperazine can give off strong fumes and catch fire if given half a chance. Forgetting to screw on a cap tightly or storing it near oxidizers can set off a dangerous situation faster than anyone expects. I remember seeing a half-empty drum get shoved next to a pile of peroxide-based cleaners. Lucky for everyone, a safety audit caught it, but the result could have been ugly.
Homopiperazine does not like warm, stuffy conditions. Heat can turn it from a solid or oily liquid into dangerous vapors. Keeping it at room temperature or cooler—not below freezing, just away from direct heat—cuts down on the chance of fumes and pressure buildup in its container. Dryness is also vital, since moisture can spark chemical changes.
Original, tightly sealed packaging offers the best defense. Metal drums with solid, chemical-proof linings usually hold up well against leaks. In smaller operations, tough HDPE bottles are common. Homopiperazine works its way through poor plastic, so the right container matters. Extra sealing with a secondary container isn’t overkill in a cluttered storeroom, especially where labels wear off or tape peels.
Warehouses often make mistakes by stacking incompatible chemicals together. The closer homopiperazine sits to acids or strong oxidizers, the greater the odds of a reaction. I’ve seen a misplaced label cause confusion that led to a recall on a whole pallet—costly and easily avoided.
The right gloves and splash goggles help, but good handling counts on alert workers and proper planning. Training goes beyond reading a safety sheet. Workers need to know what symptoms mean exposure, and how spills should actually get cleaned up—hint: avoid plain water. Skilled workers check the area for weak spots in floors or shelves before even moving the stuff.
Incidents often arise not from chemical failure, but from human error: rushing, not double-checking lids, or leaving storage rooms unlocked. From experience, assigning each chemical a dedicated spot and enforcing a strict sign-in process keeps surprise mistakes down. Digital tracking helps, but routine hands-on inspections catch the big risks.
Some folks scoff at secondary containment trays, but these trays catch leaks and spills before they snake across the floor. Ventilation—simple fans and regularly aired-out storage—stifles fume buildup. A bit of housekeeping and good labeling does more than any policy in the binder.
Homopiperazine may seem routine to those who handle chemicals every day, but its safe storage and respectful handling depend on shared effort. One person gets lazy, skips a safety check, and the whole system suffers. The best teams keep one another honest, check protocols out loud, and expect everyone to treat every chemical as if it can cause trouble. Experience counts, but constant attention counts more.
Homopiperazine pops up in chemical research and industry labs, so anyone working around it needs to show it some respect. Some folks might shrug at another intermediate, but the risks with this one shouldn’t be ignored. With my time spent in labs, it’s become second nature to double-check how we handle each chemical, but complacency breeds mistakes. Homopiperazine can irritate the skin, eyes, and respiratory system, and while it’s far from the most dangerous chemical you’ll run into, cutting corners usually makes life harder down the road.
Many incidents stem from rushing or not reading the label closely, and I’ve seen seasoned workers get caught off guard simply because instructions changed. Safety sheets spell out what can go wrong – and they offer real steps anyone can follow. Take the time to check those sheets before opening a bottle. This habit takes a minute but can save hours of discomfort and possibly a hospital visit.
Lab coats, gloves, and goggles sound basic, almost too obvious to mention. But if you’ve ever splashed even a small amount of a chemical like homopiperazine onto bare skin, you know how quickly irritation sets in. Standard latex or nitrile gloves block the chemical, and splash goggles shield the eyes. Open-toed shoes are a no-go – chemicals seep into skin faster than you think. I keep spare coats and gloves nearby because someone always forgets, and nobody wants to be the reason for a spill or reaction.
Ventilation gets overlooked in tight classrooms and makeshift labs. Homopiperazine fumes irritate airways and can build up fast in a closed space. Fume hoods aren’t just for show — they’re a safeguard against inhaling something you can’t even see. Younger lab workers, especially, seem to think holding their breath will do the trick, but vapor sticks around. If a strong smell fills the room, you’ve missed your shot at a simple fix and need to get fresh air and call for help.
Storage spaces at eye level, clearly labeled containers, and keeping incompatible materials away from each other should not be optional. I’ve watched disasters unfold because someone thought a crowded shelf could fit just one more bottle. Spacing things out and following the storage advice means you’re not hunting for the right chemical halfway through a procedure, and you’re keeping runaway spills to a minimum. Make a habit of locking up homopiperazine after use and checking caps for tight seals.
The best way to handle a chemical spill is to keep it from happening, but accidents do happen. Spill kits help here – everything from absorbent materials to neutralizing agents should be close at hand. After a spill, I’ve learned that trying to scrub down surfaces without proper tools only spreads the problem. Sweep up solids with wet towels, use ventilation, and don’t just toss rags into open trash bins. Seal them up and dispose of them as hazardous waste.
There is something reassuring about having a buddy in the lab, someone to check your back and catch mistakes you didn’t notice. Every new student learns this lesson the first time a reaction goes sideways. Homopiperazine isn’t unforgiving, but in an emergency, help from a coworker or supervisor makes all the difference – whether it’s a splash, a spill, or an unexpected reaction.
Training sessions can feel like a drag, but experience has shown me that repetition beats luck. Run drills, check eye-wash stations, and quiz everyone on where to find safety equipment. The more practice with spill kits, fire extinguishers, and emergency showers, the less panic during a real incident. The labs and workplaces that do best are the ones where these routines feel as natural as locking the front door at night.
Precaution isn’t just the responsibility of supervisors or safety officers. It belongs to everyone who sets foot near chemicals like homopiperazine. Make it personal — look out for each other, ask questions, and fix a problem before it needs a hospital visit. Trust in simple routines, and safety stops being a chore and starts being second nature.
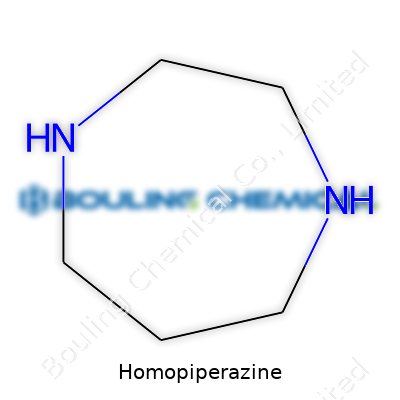
| Names | |
| Preferred IUPAC name | 1,4-Diazepane |
| Other names |
1,4-Diazacyclohexane Hexahydropyrazine |
| Pronunciation | /həˌmɒpɪpəˈreɪziːn/ |
| Identifiers | |
| CAS Number | 123-75-1 |
| Beilstein Reference | 32025 |
| ChEBI | CHEBI:28047 |
| ChEMBL | CHEMBL1236 |
| ChemSpider | 14877 |
| DrugBank | DB02994 |
| ECHA InfoCard | 19b35b08-92f6-4d8e-8807-50adbb19b6e4 |
| EC Number | 209-793-3 |
| Gmelin Reference | 47145 |
| KEGG | C06323 |
| MeSH | D006714 |
| PubChem CID | 6842 |
| RTECS number | MI7850000 |
| UNII | 1R9W0N2425 |
| UN number | UN2811 |
| Properties | |
| Chemical formula | C5H12N2 |
| Molar mass | “114.19 g/mol” |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.05 g/cm3 |
| Solubility in water | soluble |
| log P | -1.24 |
| Vapor pressure | 0.00261 mmHg at 25 °C |
| Acidity (pKa) | 8.0 |
| Basicity (pKb) | 3.80 |
| Magnetic susceptibility (χ) | -51.0e-6 cm³/mol |
| Refractive index (nD) | 1.546 |
| Dipole moment | 4.16 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 274.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | −75.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3548.7 kJ/mol |
| Pharmacology | |
| ATC code | P02CB02 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and eye irritation. |
| GHS labelling | GHS02,GHS07 |
| Pictograms | GHS06,GHS08 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | P280-P305+P351+P338-P337+P313 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | 156°C |
| Autoignition temperature | 380°C |
| Explosive limits | Explosive limits: 3.2–10.8% |
| Lethal dose or concentration | LD50 (oral, rat): 2100 mg/kg |
| LD50 (median dose) | LD50 (median dose) of Homopiperazine: "1130 mg/kg (oral, rat) |
| NIOSH | SN1225000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 5-40 |
| Related compounds | |
| Related compounds |
Piperazine Ethylenediamine Morpholine 1,4-Diazepane Piperidine |