People often overlook the humble beginnings of histidine. The story started back in 1896 when the German physician Albrecht Kossel and his student Sven Hedin first isolated histidine from protein hydrolysates. Researchers were dogged in their curiosity, slicing and dicing protein sources to discover what exactly fuels the human body and all living things. Even from my own years working in research settings, it's clear that the curiosity these pioneers had isn’t all that different from what pushes labs today: a drive to trace the tiny details that shape life. Histidine's journey from discovery to key biological building block highlights how patient observation and relentless trials lead to scientific breakthroughs. What came from painstaking research over a hundred years ago now sits in countless labs and industries, fueling both medicine and biochemistry. The historical thread tying past curiosity to today’s diversified uses proves that even small amino acids can shape how we cope with disease, nutrition, and technology.
Anyone working around proteins or animal nutrition bumps into histidine eventually. As one of the twenty standard amino acids, developers rely on it for everything—from making cell culture media to improving animal feed. Histidine earns its spot in sports nutrition as well, with athletes increasingly aware of the benefits this amino acid brings for muscle recovery and endurance. Manufacturers and formulators look for its purity and effectiveness. Those details add up, especially when the stakes are high: different industries depend on grades ranging from food to pharmaceutical, each with specific standards. Having handled formulations myself, I know the difference between medical and technical grade isn’t just academic—a missed indicator or impurity has consequences, whether for a patient or a production line. Histidine’s status as “conditionally essential” for adults and as vital for child development sends a clear signal: getting this compound right isn’t optional.
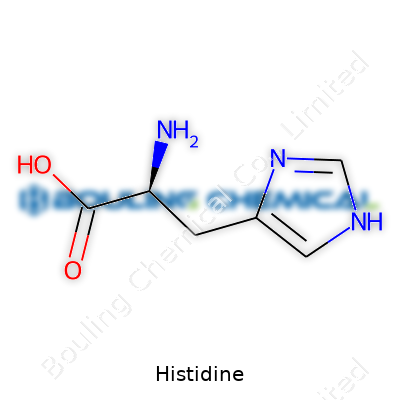
Histidine stands out with its white, crystalline powder form, easy to spot in the lab. It dissolves well in water and packs a distinct imidazole ring in its side chain, letting it play a buffer role in cells and test tubes alike. That unique structure gives it flexibility in biological systems: it acts both as a building block and a chemist’s tool. The melting point rests around 287°C (dec.), which keeps it stable through most storage and shipping scenarios. The molecule’s pKa values create an ideal environment for enzyme reactions and help maintain homeostasis where it counts. From my years mixing solutions and running reactions, the way histidine stabilizes pH never gets old—especially when it saves the day in biopharmaceutical prepping or protein purification. The hands-on experience tells you that its properties are more than just textbook trivia—they’re quietly working behind the scenes.
Regulators and consumers alike demand clear labeling and tight specs. Pharmaceutical- and food-grade histidine usually meets strict purity benchmarks, often above 98%. Labels bear details on source, batch, and lot number, as well as information on storage and allergen content. Over time, labeling laws have only gotten stricter, reflecting both growing scrutiny and respect for consumer safety. Safety data sheets spell out hazards and proper handling, often in line with REACH or FDA documentation. From my own audits in pharmaceutical settings, the labeling game isn’t about bureaucracy; it saves lives when someone on night shift knows exactly what they're handling. Technical details might look like extra paperwork, but every standard and disclosure bridges the gap between lab and real-world use.
Modern production rests mainly on fermentation using specific bacteria or yeast strains, tuned to overproduce histidine from simple sugars. Years ago, makers derived it from hydrolyzed natural proteins, but the shift to microbial fermentation opened the gates for high-purity, allergen-free batches that meet tough pharmaceutical specs. Fermentation isn’t just a buzzword—watching huge fermenters crank out what used to take days of labor from animal sources still impresses me. Downstream purification, chromatography, and crystallization ensure that nothing but clean histidine comes through. Producers screen for metals, allergens, and potential bioburden, knowing that even a small glitch could mean missing a quality mark or putting patients at risk.
Histidine’s imidazole ring gives it plenty of chemistry chops. In the lab, it acts as a catalyst and a buffer, and chemists modify it to create specialized derivatives for everything from enzyme research to radiolabeling. The ability to tweak the ring or side chain makes histidine an anchor point in peptide synthesis and a backbone for developing imaging probes and enzyme mimetics. Having learned the ropes with classic and solid-phase peptide synthesis, I’ve seen how switching one amino acid for a modified histidine can transform the activity or stability of a whole protein, landing results directly in the clinic. The range of reactions—from methylation to phosphorylation—opens new doors in drug discovery and molecular biology.
On labels or purchase orders, histidine pops up under several names. L-histidine, (S)-2-Amino-3-(1H-imidazol-4-yl)propanoic acid, and even essential amino acid mix, depending on the application. In supplements, it sits next to leucine and lysine, while in the chemical trade, it shows up under systematic or IUPAC names. Clarity in naming matters: in warehouses or pharmacies, swapping L- for D- enantiomers by accident can have real-world consequences, so keeping the paperwork precise pays off in reduced confusion and safer results.
Safety in handling histidine starts with practical details. While histidine is mostly safe, inhaling dust or eye contact can irritate, and standard personal protective equipment covers the bases. Storage conditions stick to cool, dry places, sealed from moisture. Handling protocols draw on hazards listed in the SDS, including advice on what to do in spills or accidental exposures. I’ve watched operators learn (sometimes the hard way) that skipping gloves or letting the compound soak up humidity quickly leads to wasted stock and compliance headaches. Beyond the lab, animal feed and pharma production rely on validated processes and regular audits to keep safety top-of-mind, ensuring both workers and end users stay protected.
Historically, nutrition science staked an early claim on histidine because of its role in growth and tissue repair. In hospitals, low histidine shows up in sickle cell anemia and kidney disorders, leading doctors to prescribe supplements. Industrial feed formulations use it to promote healthy livestock growth and immune function. In sports, it partners with beta-alanine to raise carnosine levels, fending off muscle fatigue. Researchers studying enzymes pull in histidine derivatives to explore catalysis, binding, and electron transfer. Formulators count on it to stabilize biologics and as a buffer in injectable drugs and vaccine manufacturing. That reach keeps expanding as scientists and product developers discover new ways to employ this reliable molecule.
Innovation in histidine research accelerates every decade, powered by new tools in structural biology, genomics, and synthetic chemistry. Focus areas include therapeutic peptides, enzyme function, and metabolic regulation. Recent work uncovers how histidine modulates immune response, coordinates trace metal ions, and influences neurotransmitter synthesis. The early guesswork in applications has shifted to targeted custom molecules and precision medicine. Lab teams now develop designer histidine analogues to probe disease mechanisms or boost the effectiveness of cancer therapies. My own dives into proteomics and mass spectrometry repeatedly circle back to histidine’s distinctive signature, a reminder of how this ancient molecule continues to shed new light on health and disease.
While histidine serves vital roles, scientists watch for toxicity risks in both humans and animal models. At typical dietary levels, side effects rarely surface. Chronic overdosing, however, sometimes triggers imbalances in trace metals, headaches, and histaminosis-like symptoms. Animal studies flagged caution over kidney and liver responses to sustained high doses, prompting tighter dosage guidelines for both supplements and clinical therapies. Toxicology panels assess everything from mutagenicity to reproductive risks, feeding data to regulatory reviews and public health advisories. I’ve read more than one study that reminds us even nutrients have limits. Prudent review of each new application helps keep benefits ahead of risks—a lesson the supplement industry has learned as more products enter the global market.
Looking ahead, histidine keeps gaining momentum in biotech and medical science. Synthetic biology teams design new metabolic pathways using histidine, aiming for greener, more efficient fermentation. Drug developers work up histidine-derived compounds as enzyme inhibitors and imaging agents. Global trends in personalized nutrition and sports supplementation keep product managers and researchers busy responding to evolving consumer demand. Even artificial intelligence joins in, using machine learning to predict histidine interactions and optimize formulations. My time consulting for life science startups taught me that the next idea rarely comes from a vacuum; the best innovations borrow from years of steady progress—histidine’s story included. As the world faces tougher demands for safer, more effective therapies and sustainable production, histidine stands ready for its next chapter.
Most people don’t spend their afternoons wondering about amino acids, but histidine deserves a place on the radar. This essential amino acid helps build proteins and keeps important chemical processes going. It’s involved in growth, tissue repair, and making neurotransmitters like histamine – the same one that’s behind allergy symptoms and stomach acid production. Human bodies can’t produce enough histidine from scratch, so regular intake through food or supplements matters, especially during growth, recovery, or medical conditions.
Some athletes and gym regulars take histidine supplements hoping to reduce fatigue. Muscle tissue stores carnosine, made from histidine and beta-alanine. Studies show carnosine can buffer acid in muscles, letting you push harder before burning out. In several trials, folks taking histidine and beta-alanine together notice better endurance and shorter recovery times. While eating a balanced diet covers these needs for most people, vegetarians and older adults sometimes fall short, making supplements more relevant.
Another interesting angle is inflammation. Early research connects histidine to anti-inflammatory benefits, possibly because of its influence on histamine. People with chronic kidney disease often have low histidine levels, which can make inflammation or anemia worse. Small studies experimenting with supplements show promising signals for lowering inflammation markers. The science still needs bigger trials, but the basic mechanism lines up with what doctors see in practice.
There’s talk about histidine’s role in mental well-being, with links to neurotransmitter balance and stress response. Depression and anxiety patterns often relate to nutrient status. An observational study in Japan tracked hundreds of adults and found that those with higher dietary histidine reported more stable moods and fewer signs of psychological distress. It’s not a magic bullet for depression, yet it highlights how nutrients tie into mental health – often overlooked outside specialist circles.
Since histidine turns into histamine, which drives allergy symptoms, people sometimes worry about taking too much. For most, the body keeps a tight handle on conversions, and supplementation at reasonable levels won’t turn you into a sneezing mess. In fact, a healthy supply of histidine supports immune cells and helps them respond to invaders properly. Some rare genetic disorders prevent histidine from breaking down efficiently, so folks with those conditions should steer clear of extra supplementation without medical advice.
Standard food sources like meat, dairy, beans, and whole grains provide histidine in forms your body knows how to use. Supplements bring value when prescribed for specific deficiencies or during periods of rapid growth or recovery. Too much histidine can interfere with other amino acids and cause gastrointestinal upset or headaches. Anyone considering a supplement should check with a healthcare provider, especially if living with health conditions or taking medications that affect the immune or renal system.
While histidine supplements hold promise for exercise recovery, inflammation, and some mental health aspects, most of us meet our needs through a varied, protein-rich diet. For people with higher needs – athletes, older adults, or those managing chronic illnesses – thoughtful supplementation under guidance can help fill gaps. Real health improvements come from understanding your body’s needs, prioritizing whole foods, and relying on supplements when evidence and medical experience support their use.
Histidine plays a key part in making proteins and supporting growth and repair within the body. This essential amino acid helps produce histamine, feeds the immune system, and keeps tissues strong. We get histidine from foods like meat, dairy, whole grains, and nuts. Most people eating a balanced diet hit the daily mark without even trying. Still, supplement bottles line grocery shelves, promising brain, gut, or immunity boosts. So it’s fair to wonder if daily histidine supplements cause harm or if they hold real benefits.
The average adult needs about 8 to 12 milligrams of histidine per kilogram of body weight each day. For most, regular meals cover this without effort. Studies show that even at higher doses, histidine rarely leads to toxicity in healthy folks. The European Food Safety Authority and U.S. National Institutes of Health haven’t set a strict upper safe limit for histidine either, since few problems turn up at reasonable doses.
One thing people forget is that more doesn’t always mean better. Large doses of any amino acid can upset balance, outpace kidney function, or lead to digestive troubles. In rare situations, histidine can raise histamine levels in the body, leading to headaches, rashes, or allergy-like symptoms. My own experience working in community healthcare tells me that side effects look much more likely when people take these supplements on top of an already rich diet. Instead of raising energy, some folks report brain fog or mood swings. These are reminders that the body likes its nutrients in the right amounts—not overloaded.
Some research circles suggest that folks with chronic kidney issues or rare metabolic conditions may see high histidine levels build up, so doctors advise caution. Pregnant women, breastfeeding mothers, and those with liver troubles should always ask their healthcare provider about extra amino acids. One fact never changes—kids need to get enough protein, not just one amino acid, to keep growing.
For the average healthy adult, adding a histidine pill to the morning routine rarely tips the balance in a meaningful way. In special cases, such as inherited disorders or after discussion with a doctor, supplementation helps fill gaps. In my years advising patients, I noticed vegetarians and vegans sometimes ask about amino acids since plant sources often contain less histidine. Still, a good mix of beans, lentils, nuts, and grains generally covers the body’s needs just fine. Supplements become relevant after a full dietary review and blood work.
Whole foods beat powders or pills in providing the range of nutrients needed to function best. Unless a lab test says otherwise or a professional recommends it, most people do fine with food sources of histidine. Shop with an eye for colorful, fresh choices—not just after a supplement quick fix. Anyone thinking about regular supplementation, especially those already on other medications or living with chronic illness, should talk with their healthcare provider first. Responsible health decisions take context, conversation, and careful review.
Histidine is one of those amino acids you won’t hear about at every gym locker or health food section, but the body uses it in everyday processes: muscle growth, tissue repair, and making brain chemicals like histamine. The question comes up, especially for folks fiddling with supplements or adjusting their diet: How much histidine does a healthy person need each day?
Research shows adults benefit from about 8 to 12 milligrams of histidine per kilogram of body weight daily. For a grown person weighing 70 kilograms, that runs somewhere between 560 and 840 milligrams each day. It’s not some random number — several nutrition organizations landed here after watching groups of healthy volunteers, checking their blood levels, and paying attention to symptoms when people don’t eat enough.
Children actually need a bit more per body weight because they’re growing. Infants get around 28 milligrams per kilogram daily from breast milk or formula. Pregnant and breastfeeding women also need higher amounts since their bodies work overtime building and feeding a baby.
In my experience, most people making reasonable food choices get enough histidine without trying. Dairy, chicken, fish, soy, meat, eggs, and nuts–if you have even a handful of these through the week, you’re probably set. For folks following a strict vegan plan, histidine comes in soy, beans, lentils, and whole grains. So unless someone is on a seriously restricted diet or dealing with a gut absorption issue, extra pills or expensive powders usually don’t solve a real problem.
Supplements sometimes tempt people who read about stamina or skin healing online. Some companies promote doses far above science-backed recommendations, hinting at all sorts of benefits. Science hasn’t confirmed these claims. Going beyond what your body needs won’t speed up muscle gains, cognitive skills, or immunity. Too much histidine from supplements turns into higher levels of histamine, sometimes causing headaches, flushing, or digestive trouble.
Rare genetic disorders or metabolic diseases sometimes require careful protein balancing, so a doctor, dietitian, or metabolic specialist sets the dose. Folks with kidney or liver disorders should not tinker with amino acid supplements without expert advice. For the average adult just looking for a health boost, though, more isn’t better; it can lead to imbalances and new symptoms.
Skipping routine bloodwork won’t show a histidine shortfall in healthy people. For almost everyone, focusing on steady protein sources through meals handles the need. I often tell people to keep their protein rich but varied, not doubling up on shakes or pills. If you feel off, have unexplained symptoms, or suspect a deficiency, ask a qualified doctor or registered nutritionist for a review. They can spot patterns and offer real solutions without guesswork.
Healthy eating patterns rarely need single-amino supplements, unless a diagnosed illness limits protein or amino acid absorption. Spending money on extra pills may just leave your wallet lighter. Trust the basics: variety in diet, science-backed guidelines, and honest conversations with health professionals.
Histidine is one of those amino acids that gets far less attention compared to big names like lysine or tryptophan. Found in meat, dairy, grains, and produced in small amounts by the body, it plays a part in building proteins and helps form histamine, which deals with immune responses and digestion. Because of that, people sometimes see supplements pop up in health stores, often marketed for allergies or boosting energy.
Most people eat foods rich in histidine without experiencing problems. The body knows how to handle this nutrient, and uses what it needs. With supplements, the story shifts. Taking more than the body can use sometimes brings out headaches or mild digestive upset. Some folks might notice nausea or a drop in appetite, especially if they start with higher doses right out the gate. This isn’t unique to histidine—many supplements, even herbal teas, can stir up a sensitive digestive system if the dose is too high.
The place where histidine gets tricky involves medications and other supplements. Histidine helps make histamine—a compound that plays a role in allergic responses and stomach acid production. In my experience chatting with pharmacists and doctors over the years, concerns come up for people who use medications that affect histamine levels. For example, those on antihistamine drugs for allergies or acid reducers like famotidine. Adding more histidine into the mix could blunt the effect of these drugs, or just make side effects more likely, such as drowsiness or heartburn.
Certain drugs for immunity—like corticosteroids—or medicines that influence metal absorption in the gut can also tangle with histidine. It binds to trace metals in the intestine, and large doses may mess with zinc or copper absorption. The science isn’t settled, but it’s another reason to check in with a healthcare provider before rushing into new supplements. For cancer patients, some chemotherapy medicines interact with amino acids. Any new supplement deserves extra scrutiny for folks living with serious conditions.
Kids and pregnant women have higher protein needs, but adding a histidine pill probably won’t offer much benefit. For these groups, the safety data just doesn’t exist in any meaningful way. Relying on a balanced diet stacked with beans, fish, and seeds covers the bases, with fewer worries about skewing nutrient levels. People with kidney or liver issues face another hurdle. Since amino acids get processed by these organs, overdoing any supplement risks more pressure on already overworked systems.
Relying on food for histidine makes the most sense for most people. Years working with nutritionists taught me that supplements bring value in specific, diagnosed deficiencies—usually under medical guidance. For the average person, just eating a varied diet keeps levels in a healthy range. If someone feels a need for more, testing blood amino acid levels before starting anything high-dose can dodge unnecessary problems. Checking medication and supplement labels for histidine content helps spot potential interactions early.
Staying curious about nutrients matters. Even something as simple as an amino acid carries ripple effects in the body, especially when taken outside the dose nature intended. Honest discussion with doctors and reviewing current medications keeps things on track. For now, keeping histidine as part of an overall balanced diet, respecting how it interacts with drugs and the body, offers the surest path forward with the fewest risks.
Every allergy season, people hunt for anything that might ease the sneezing and itchy eyes. Lately, some folks have turned their attention from pills and inhalers to something found right in food: the amino acid histidine. Its name pops up because of its link to histamine, that troublemaker behind runny noses and swelling eyes. Foods like meat, fish, and soy contain histidine, raising an obvious question: does eating more of it help—or hurt—people who struggle with allergies or immune issues?
Histidine stands out because the body turns it into histamine. Most allergy sufferers hear “histamine” and imagine breakouts or wheezing, but the story runs deeper. The immune system uses histamine to fight off things that might harm us, though it sometimes goes overboard. On paper, it seems like more histidine could stir up more histamine, making things worse for allergy-prone folks.
Researchers have looked at this idea for a long time. One study in “The Journal of Allergy and Clinical Immunology” checked whether adding or reducing histidine changed people’s allergy symptoms, but results stayed mixed. A healthy young adult will not see a big spike in histamine levels just from eating more protein-rich foods. The body has a way of holding the line. That means diets with higher histidine usually don’t cause dramatic changes in allergy symptoms for most people. Serious allergy attacks usually result from triggers like pollen, dust, or certain foods, not from a plate of salmon.
Some research teams chase a more complicated angle, focusing on histidine’s role outside allergies. In the immune system, histidine supports healing and cell growth. Lab studies indicate that animals on low-histidine diets heal slower and catch infections more easily. For people going through stress, heavy exercise, or illness, keeping up protein intake—including histidine—matters more.
Plenty of everyday foods provide all the histidine a body needs. Meat, fish, eggs, nuts, seeds, and many beans carry more than enough. Research shows most adults in the U.S. and Europe already get enough through regular meals. So a lack of histidine doesn’t pose much risk for the average person—unless severe undernutrition is happening.
Many with allergies or immune problems start searching for diet changes hoping for relief. Protein—especially certain amino acids—matters a lot, but so do rest, stress management, and regular health check-ups. Avoiding known allergens always beats hoping a small dietary tweak like extra histidine can erase strong reactions. Over-the-counter antihistamines target histamine head-on, blocking its action fast. No clinical trials show that eating more histidine matches those benefits.
Doctors sometimes recommend restricting high-histamine or histamine-liberating foods for people who experience “histamine intolerance,” though this remains rare. Symptoms like headaches, rashes, and hives may improve once foods like aged cheese or fermented products leave the plate. Even with that, histidine alone doesn’t drive this process.
The hype around supplements often outpaces facts. Most people gain nothing from adding histidine capsules to their routine, and focusing on whole food sources covers every need. True, histidine does matter for immune health, but not as a allergy cure-all. Anyone thinking about making big changes would do better talking with a registered dietitian or doctor familiar with their story and medical background. That’s how to sort out real solutions from empty promises.
| Names | |
| Preferred IUPAC name | 2-amino-3-(1H-imidazol-4-yl)propanoic acid |
| Other names |
L-Histidine His 2-Amino-3-(1H-imidazol-4-yl)propanoic acid |
| Pronunciation | /ˈhɪstɪdiːn/ |
| Identifiers | |
| CAS Number | 71-00-1 |
| Beilstein Reference | 12080 |
| ChEBI | CHEBI:5776 |
| ChEMBL | CHEMBL644 |
| ChemSpider | 54612 |
| DrugBank | DB00117 |
| ECHA InfoCard | 100.000.133 |
| EC Number | 3.5.4.8 |
| Gmelin Reference | 5164 |
| KEGG | C00135 |
| MeSH | D006641 |
| PubChem CID | 6274 |
| RTECS number | MM1400000 |
| UNII | JQS6WI9V6P |
| UN number | UN3334 |
| Properties | |
| Chemical formula | C6H9N3O2 |
| Molar mass | 155.154 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.59 g/cm³ |
| Solubility in water | 43 g/L (20 °C) |
| log P | -3.32 |
| Acidity (pKa) | 6.0 |
| Basicity (pKb) | 6.04 |
| Magnetic susceptibility (χ) | -33.1×10⁻⁶ cm³/mol |
| Viscosity | 1.44 cP |
| Dipole moment | 6.14 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 159.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1323.5 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3015.6 kJ/mol |
| Pharmacology | |
| ATC code | A16AA06 |
| Hazards | |
| Main hazards | May cause eye, skin, and respiratory tract irritation |
| GHS labelling | GHS07, GHS08 |
| Pictograms | FFMHH |
| Signal word | Warning |
| Hazard statements | Hazard statements: Not a hazardous substance or mixture according to Regulation (EC) No. 1272/2008. |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Autoignition temperature | 411 °C |
| Lethal dose or concentration | LD50 (rat, oral): 12 g/kg |
| LD50 (median dose) | LD50 (median dose) of Histidine: 12,000 mg/kg (rat, oral) |
| PEL (Permissible) | Not established |
| REL (Recommended) | 35 mg/kg BW |
| Related compounds | |
| Related compounds |
Histidinol Histidine methyl ester Histidyl Hydroxyhistidine |