People often look to the past to find out how a simple molecule like ethylmorpholine found its way into research, manufacturing, and even daily life. Back in the early to mid-20th century, chemists started exploring morpholine derivatives, aiming to fine-tune properties like solubility and reactivity. Ethylmorpholine didn't show up by accident; its development followed the needs of chemists hunting for new intermediates and solvents. Laboratories in Europe and the United States realized that adding an ethyl group to morpholine unlocks slightly different chemistry—something that traditional amines or unsubstituted morpholines couldn’t offer. By the 1970s, commercial catalogs started listing ethylmorpholine as a specialty chemical, and since then, it has trickled into pharmaceutical, agrochemical, and materials research.
Walk into a lab supply store—if you know what to ask for, you'll spot ethylmorpholine as a clear, nearly colorless liquid. You usually see it bottled up in sturdy amber containers that block out ultraviolet light, which can mess with many organic amines. Each bottle ships with detailed labeling about percent purity, lot number, safety data, and, if you’re lucky, a certificate of analysis spelling out exactly where that batch came from. People who work with this compound often care a lot about purity; a hint of water or other amines can change a whole experiment’s results.
Most chemists know right away from its sharp, ammonia-like smell that they’re dealing with a strong base. Ethylmorpholine brings a molecular formula of C6H13NO, weighs in at about 115 grams per mole, and holds a boiling point between 137 and 141 °C, which means it handles moderate heat but isn’t as hard to distill as some larger molecules. It dissolves easily in both water and organic solvents. The ethyl group at the nitrogen gives it different steric and electronic properties compared to parent morpholine—you get less hydrogen bonding, more hydrophobicity, and changes in basicity. That means it works in places where you want a slightly less sticky or more oil-loving amine.
Manufacturers don’t hide the details on the label. You’ll spot precise concentration ranges, sometimes 98% or better, water content down to tenths of a percent, and sometimes residual solvents pressed below industrial limits. Storage directions encourage keeping the liquid tightly capped, far from sunlight and strong oxidizers. Barcodes and QR codes now show up more often than not; these help trace every batch back to its origin. Regulators call for hazard pictograms, exposure limits, and detailed first aid instructions, reflecting the compound’s alkaline nature and potential risks.
Ethylmorpholine gets made mostly by alkylating morpholine with ethyl halides—think ethyl bromide or ethyl chloride—in the presence of base. The reaction chews through morpholine, and you skim off the product by distillation. Some factories swap out halides for dimethyl sulfate or diethyl sulfate, boosting yields and making purification easier. Operators watch the process to limit over-alkylation, which would throw extra ethyl groups onto the nitrogen or other parts of the molecule, creating a blend of side-products that no one really wants. Industrial scaling relies on stainless steel reactors, good temperature controls, and effective distillation setups.
Researchers rely on ethylmorpholine for nucleophilic substitutions, often using the nitrogen as a building point for larger structures. That lone pair on nitrogen reacts with alkylating or acylating agents, creating a toolkit for building bigger, branched molecules. Chemists sometimes oxidize ethylmorpholine to chase after N-oxides, which behave quite differently for catalysis or as intermediates. Under acidic conditions, you can quaternize the nitrogen, while under oxidizing conditions, you can even swap in function groups to turn it into a backbone for ligands in coordination chemistry. Its flexibility on the lab bench makes ethylmorpholine a familiar face in many synthesis plans.
Looking through chemical catalogs and research papers, you’ll see ethylmorpholine pop up under a few names—1-ethylmorpholine, N-ethylmorpholine, and C6H13NO among them. European suppliers sometimes stick to morpholine, N-ethyl-, while others just list the CAS number, 100-74-3, for quick searching. Industry shorthand rarely misses its official name, since confusion with other morpholine derivatives could lead to trouble in an experiment or manufacturing process.
No one who’s handled amines ignores the precautions; ethylmorpholine stings on contact and burns in most eyes or mucous membranes. Industrial safety sheets urge wearing gloves, goggles, and working under proper ventilation. The EPA and European chemical regulators set limits on exposure, especially in manufacturing where vapor concentrations ramp up fast. Companies teach operators emergency clean-up steps and spill response, and storage lockers always keep the material away from acids or oxidizers. Transport containers carry hazard codes and emergency contact info. People who cut corners on these rules quickly face expensive mistakes—health-wise and regulatory-wise.
Over the past decades, labs began using ethylmorpholine for specialized organic synthesis, especially where classic morpholine fell short for solubility or compatibility. Formulators working in industrial coatings, lubricants, or corrosion inhibitors sometimes turn to it for its balance of basicity and altered hydrophobic profile. Drug research found ways to tuck ethylmorpholine into side chains or scaffolds, chasing better pharmacokinetics or stability. It doesn’t carry the widespread fame of other amines, but it holds a steady place for niche applications. Occasionally, you’ll find pilot plant engineers exploring ethylmorpholine-based catalysts, driven by the way its structure interacts with metals or reactive centers in chemical transformations.
Plenty of innovation centers around modifying ethylmorpholine’s backbone to chase new traits. Researchers in medicinal chemistry test new derivatives against microbes and cell lines, hoping to spot leads with lower toxicity or longer half-lives. Environmental scientists investigate its breakdown in water, looking for byproducts that might affect aquatic systems. New patents show up involving its use as a ligand in metallorganic frameworks or a starting point for designer surfactants. Academic papers highlight tweaks—substitution patterns on the ring, swapping in heavier alkyl groups, or appending functionalities that create novel materials. Industry trends suggest ongoing interest, often tied to evolving standards in green chemistry and lower-emission processes.
Everyone taking ethylmorpholine from the bench into a real-world setting wants to know what happens when it lands on skin, enters the water supply, or gets inhaled. Toxicology studies in animals outline moderate acute toxicity, with pronounced irritation on first contact. Chronic exposure brings potential for kidney and liver stress, tested both in animals and on human cell cultures. Safety data highlights risks of eye damage, and the material safety data sheets flag possible effects on aquatic systems in case of spills. Researchers work to balance those risks against the value of the compound, looking for ways to neutralize spills and treat waste streams effectively. Regulatory agencies require clear documentation of metabolism and breakdown products, especially before approving new pharmaceutical applications.
Ethylmorpholine stands at a kind of crossroads, as regulatory pressure builds for safer, greener, and more sustainable chemicals. More researchers aim to design derivatives that lower environmental impact without dropping the chemical versatility users depend on. Others seek production routes that cut emissions or use renewable starting materials. Companies might scale back use in some applications, but curiosity continues in catalysis, advanced materials, and new drug candidates. Smart process improvements and careful management could unlock new areas, moving ethylmorpholine from specialty corners into more mainstream use, provided it can clear the hurdles of safety and sustainability.
You don’t hear folks talk about ethylmorpholine around the dinner table. Still, this colorless liquid has a habit of turning up where the average person least expects it. Chemists and industry workers have relied on it, though rarely does its name make headlines. In my years keeping tabs on chemical safety and supply chains, I’ve seen ethylmorpholine’s roles stretch further than most realize.
Take a walk near a rubber processing plant and you might pass by trucks hauling ethylmorpholine. Companies lean on it for vulcanization—a key process that turns soft, sticky rubber into the tough tires and weather-proof seals we count on every day. Ethylmorpholine acts as what the industry calls an “anticaking agent.” Without it, you’d deal with powdery ingredients clumping up like flour gone bad in a humid pantry. Workers end up with fewer messes, and factory machines don’t stall out because of sticky blockages. Rubber shapes, rolls, and stretches the way it should.
In agriculture, this chemical finds a solid place as a builder for herbicides and pesticides. Whether folks like it or not, farms in many countries face tough weeds and stubborn pests. Researchers develop tools for fighting them off, and chemists blend ethylmorpholine into those formulas. The goal isn’t to make headlines. The aim is to keep fields productive, even as climates and market demands shift.
Very few people outside of pharma labs discuss chemical intermediates like ethylmorpholine. Behind the scenes, though, it’s part of the recipe that produces medicines. Think of it like a sous chef—not the star of the dish, but without it, the meal falls flat. Drug companies use ethylmorpholine during synthesis because it connects other pieces together or changes the way molecules behave.
I’ve come across a fair share of reports raising flags about chemical runoff and exposure. Every chemical—ethylmorpholine included—invites responsibility. Factories need systems that keep leaks out of creeks and air systems. Workers must suit up and use gloves, goggles, and good ventilation. The goal? Handle ethylmorpholine smartly and avoid health issues down the line. It’s not just about the people mixing the chemicals. Local water, wildlife, and wider communities all expect safe handling.
It’s clear that the world leans on specialized chemicals, but complexity grows as more industries expand in crowded regions. Safer substitutes exist for some uses, although transition comes at a price. Some companies now explore less hazardous anticaking methods for rubber or review alternative chemical pathways for drug synthesis, looking to reduce reliance on older reagents whenever possible. Real progress takes both investment and regulatory pressure; industry groups don’t just change out of goodwill.
Folks outside the lab rarely hear the stories of how things get made, but substances like ethylmorpholine sit behind so many everyday conveniences. Paying attention to the paths these chemicals travel—and encouraging safer processes—matters if you care about both modern products and the planet’s future.
A lot of chemicals get thrown around in labs and discussions, but ethylmorpholine grabs interest for good reasons. Its formula, C6H13NO, isn’t just a random string. Each letter and number speaks to the way scientists have learned to build new tools for medicine, industry, and research. For folks mixing up solutions, running pharmaceuticals, or just wrestling with organic molecules, boiling it down to C6H13NO strips away the confusion.
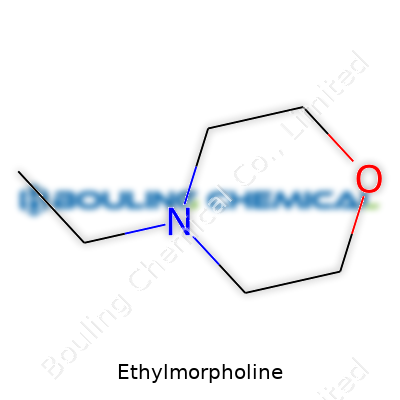
Let’s talk through what those letters mean. Six carbons, thirteen hydrogens, a single nitrogen and an oxygen. This combination is more than naming rights. Scientists structure these elements to fashion a ring—the morpholine part—while tacking on an ethyl group (that’s two carbons, just hanging out the side). That small tweak changes how the molecule behaves, both in the body and in the lab. If you spent enough time under a fume hood, you start to respect how swapping out a methyl for an ethyl can flip a compound’s abilities.
Some folks picture chemistry as endless rows of bottles nobody can pronounce, but once you see how a small chemical formula influences things, the story gets interesting. Ethylmorpholine serves as a building block in pharmaceuticals and acts as a catalyst in specialty reactions. That means it shows up in the background of compounds that help treat illness or speed up tricky steps in industrial processes.
It isn’t only about big industry or medicine. High school chemistry labs reel in young science fans every year with demonstrations using substances just like ethylmorpholine. It doesn’t take much of an imagination to understand how these chemicals become everyday problem-solvers, especially when time and efficiency matter.
Handling a compound like ethylmorpholine brings safety into focus. Nitrogen-containing rings don’t always play nice with human tissue, and many folks in the field remember their first chemical splash with more than a little discomfort. Direct contact can cause irritation. Inhalation could send you coughing and looking for fresh air. It’s a good reason to wear gloves, keep hoods on, and never be in a hurry with transfers or mixes.
Waste management becomes another issue. Pouring leftovers down a drain isn’t smart. A better route—collecting used chemicals for proper disposal—not only keeps the environment safer but saves headaches down the line. That came up during my first year as a lab technician, and those lessons stuck longer than any lecture.
Careful labeling, improved training, and easy-to-understand safety manuals always lower the risk for lab workers. Sometimes, simply slowing down and double-checking bottles or formulas keeps accidents from happening, especially when several compounds look or sound alike. Substitution can come into play for some applications—a safer cousin molecule might cut risk and still offer the same payoff for a reaction or synthesis.
So much rides on knowledge that goes beyond formulas. Ethylmorpholine’s details may seem small, but those details ripple out into safer labs, smarter industrial practices, and better products. Focusing on one compound demonstrates how science intersects real life, turning a row of letters and numbers into something truly useful.
Ethylmorpholine pops up in a handful of chemical processes and research labs, but outside the scientific circles, not many folks have even heard the name. Those who have might wonder just how safe it is to have around. I’ve spent enough time in labs and around manufacturing floors to know real caution beats wishful thinking every time.
You don’t run across ethylmorpholine in daily life—certainly not in a grocery store or hardware aisle. It lands mostly on lab benches and in chemical plants. Folks wearing goggles and gloves handle it, not because they like dressing up, but because they know what they’re working with. The stuff doesn’t ask for much attention, but that quiet exterior can throw off newcomers.
Ethylmorpholine carries a reputation in the chemical community. People who work with it have seen warnings about severe eye and skin irritation. Vapors floating around can irritate the respiratory system, and in a packed workspace, eyes and throats end up sore pretty quick. Accidental spills happen. Some of the old timers in the business remember younger workers skipping gloves, only to get redness and burns on their hands by lunch.
The long-term stories make you pause a little longer. Prolonged exposure seems linked to problems nobody’s in a hurry to sign up for—damage to the liver and kidneys has shown up in animal studies where it’s been tested in bigger doses. Now, most work sites won’t see those concentrations, but the lesson holds: trust the research, not your gut.
On the toxicity chart, ethylmorpholine isn’t at the top, but it doesn’t sit down at the bottom, either. Swallowing it accidentally is a bad idea, as it can cause nausea and even some neurological symptoms if a lot gets into the system at once. Pour it out where it shouldn’t go, and it won’t just disappear. Runoff that lands in water can harm aquatic life, and it takes a while for the environment to break it down.
Companies and researchers keep mentioning personal protective equipment and ventilation, and that’s not just legal language. From experience, even a well-designed hood doesn’t feel like overkill for chemicals like this. Proper labeling saves everyone headaches, especially as bottles age and paint starts to chip.
For workers, the rule book stays simple if taken seriously: gloves, goggles, coats, and working upwind from open containers if possible. Anyone tasked with transporting or storing it learns pretty quick to check seals and secondary containment. Training always beats shortcuts.
Some places invest in spill kits and train everyone from top to bottom, making fast cleanups standard. Waste management firms usually know the right routes—sending ethylmorpholine down the drain is a rookie mistake, and fines come quick for that.
Research keeps rolling forward, searching for safer alternatives or smarter ways to work with tricky chemicals like ethylmorpholine. Green chemistry practices have already reduced risks in plenty of labs by swapping out more hazardous stuff with milder substitutes. It all comes down to respecting the material, learning from those who handled it before, and never letting up on safety.
Ethylmorpholine doesn’t get the attention of household names like bleach or gasoline, but its risks feel just as real to those who’ve worked among flammables and corrosives. Anyone who's spent time in a lab or chemical plant knows that treating such materials lightly invites trouble. Vapors build up. Skin burns show up long after a splash. As someone who once walked into a warehouse where solvent containers leaked fumes all day, I remember what anxiety smells like — sweet and sharp with a hint of panic. The lesson stuck: careless storage costs safety.
Ethylmorpholine calls for respect around heat. Leave a drum near radiators or a sunny window, and you’re gambling. Fire doesn’t send warnings before it roars. A cool, shaded room away from any hint of ignition helps everyone breathe easier. There’s science behind this. The flash point of Ethylmorpholine sits at about 38°C (100°F), which slides under common summer nights in some places. The point isn’t just to “keep cool”; it’s about dodging the sort of mistake that leads to headlines and hospital visits. Refrigeration seems extreme, but that approach often beats regrets with chemicals like this.
Dampness invites nasty reactions, both for the product and anyone cleaning up the mess later. Once, I popped open a container after a humid week; inside, a goopy residue had started to line the cap. Water in the air may not spark fires, but it contaminates, eats away labels, and even kicks off chemical changes. Keeping Ethylmorpholine sealed tight and far from humid air pays off by allowing the contents to stay intact — every time you break the seal, you’re making a choice between quality and compromise.
Oxygen doesn’t play nice with volatile materials either. Even with a plain screw cap, some vapor will tug at the lid, and eventually, corrosion or pressure marks its territory. I’ve learned to prefer containers with a tight seal, either PTFE-lined or stainless steel, since the old tin cans often stain and rust. Never underestimate what a slow leak can do — fumes drift, and headaches set in before anyone realizes what went wrong.
Shelving looks easy until someone stores acids, peroxides, or oxidizers side-by-side with Ethylmorpholine. An old co-worker used to say, “A disaster needs only a shelf.” He wasn’t wrong. Those busy doing inventory sometimes cut corners, brushing a new bottle right against something reactive “just for a moment.” That moment can spiral into a chain of events set off by one tilted bottle or an accidental spill. The answer isn’t another training video; it’s a culture of double-checking and a willingness to question even a senior’s shortcut.
Most advice on chemical storage leans on regulations and datasheets. These matter, sure, but safety really takes root in habits. Use every sign, every checklist, and every lockable cabinet available. Don’t store more than you need — large reserves drive up risk and invite neglect. Walk through the storage area each week as if a stranger, asking, “Would this setup keep me safe if something spilled or caught?” I’ve saved more headaches catching little leaks and faded labels than any inspection ever did.
People rarely talk about the small victories: that day the spill kit got used, the time a missing label meant stopping and asking before pouring. These add up. Ethylmorpholine demands respect, not fear, and a promise never to rush the job. It’s a commitment written in clear labels, cool rooms, dry shelves, and a culture where questions always come before cleanup.
Anybody who’s worked around labs or factories knows that Ethylmorpholine isn’t something you want to take lightly. I remember my early days at a chemical processing plant—some folks would rush through the motions, but the risk you carry with something like this always makes you slow down and respect the rules. This compound doesn’t play nice with skin or lungs, and spills can cause headaches that stretch well beyond cleanup.
Ethylmorpholine gives off a sharp odor that hits you before you even uncap the bottle. It bites at your nose and seems to cling to your clothes until you air out. If you've ever splashed acetone or formaldehyde anywhere, this stuff feels similar but sometimes more aggressive. Standard cotton gloves sometimes lose their edge with repeated contact. Nitrile gloves, those tough little blue shields, stand up much better. Goggles aren’t optional, they’re just what you grab before opening any container.
Spills are another story entirely. I’ve yet to meet a floor that likes Ethylmorpholine. Porous surfaces gulp it down, so you need to get an absorbent material on it fast—nothing fancy, just plenty of granules or pads that handle solvents. Ventilation matters. You can open windows or crank up a fume hood, but if you’re in an old building or basement lab, an extra portable fan helps. Trust your nose; if you smell it, you’re probably not as safe as you think.
Some of the worst days at work followed after someone left the storage cabinet unlocked or ignored the safety data sheet. Ethylmorpholine seeps through open containers, and even a small puddle brings out rashes and watery eyes like clockwork. Inhaling vapors for even half an hour can mean needing fresh air and a sit-down for the rest of your shift.
There’s research backing this up—regular exposure causes skin and eye irritation, respiratory discomfort, and sometimes nausea. The U.S. National Library of Medicine and Chemwatch both lay out records of similar mishaps. Even outside a lab, anybody transporting this material needs decent containers, clear hazard markings, and a plan for leaks. To ignore these facts corners you into relying on luck. The facts don’t care if you’re running late or under pressure to finish up.
I’ve always respected teams that put routine over shortcuts. Clear labeling stands at the front of that. No half-torn stickers or faded pen marks—every bottle, jar, and tanker should scream what it holds. The Safety Data Sheet shouldn’t get buried in a drawer; keep it posted somewhere obvious. Someone on each shift needs to know exactly where the eyewash station, showers, and spill kits wait.
Disposal practices keep everyone around safe, not just those working hands-on. Bins marked for hazardous waste take only compatible chemicals, and transport happens in bundles. You carry out storage in a cool, dry place, with the right kind of fire extinguisher on standby. Flammability sits near the top of the concern list—not as quick to ignite as petrol, but one spark and the situation escalates.
If you’re new, learn from every little mistake—wipe up, wash out, record it, ask for guidance. If you’ve been around, teach by example; talk through the close calls, share why the rules exist, and notice gaps in the routine. It comes down to respect for yourself and the strangers who might share your space. Facing Ethylmorpholine means relying on effort, teamwork, and not pretending luck lasts forever. These everyday steps shape safety one shift at a time.
| Names | |
| Preferred IUPAC name | 4-ethylmorpholine |
| Other names |
EM N-Ethylmorpholine 4-Ethylmorpholine |
| Pronunciation | /ˌiːθɪlˈmɔːrfəˌliːn/ |
| Identifiers | |
| CAS Number | 100-74-3 |
| Beilstein Reference | 24407 |
| ChEBI | CHEBI:77515 |
| ChEMBL | CHEMBL24037 |
| ChemSpider | 16715 |
| DrugBank | DB08797 |
| ECHA InfoCard | 03b7c9e5-0a73-44da-ae4b-2f7a3d1e0c12 |
| EC Number | 203-724-3 |
| Gmelin Reference | 113840 |
| KEGG | C19319 |
| MeSH | D004995 |
| PubChem CID | 77833 |
| RTECS number | QD0700000 |
| UNII | 8E8R8K715F |
| UN number | 2810 |
| Properties | |
| Chemical formula | C6H13NO |
| Molar mass | 129.21 g/mol |
| Appearance | Colorless to yellowish liquid |
| Odor | amine-like |
| Density | 0.928 g/mL at 25 °C (lit.) |
| Solubility in water | Miscible |
| log P | 0.32 |
| Vapor pressure | 0.5 mmHg (20°C) |
| Acidity (pKa) | 8.3 |
| Basicity (pKb) | 5.10 |
| Magnetic susceptibility (χ) | -62.5e-6 cm³/mol |
| Refractive index (nD) | 1.427 |
| Viscosity | 0.87 cP (25°C) |
| Dipole moment | 3.06 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 348.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -285.4 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4246 kJ/mol |
| Pharmacology | |
| ATC code | N02AB05 |
| Hazards | |
| GHS labelling | GHS02, GHS05, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H314 |
| Precautionary statements | P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2-3-1-△ |
| Flash point | 46 °C |
| Autoignition temperature | 215 °C |
| Explosive limits | 1.5–8.8% |
| Lethal dose or concentration | LD50 oral rat 256 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 242 mg/kg |
| NIOSH | KN5075000 |
| PEL (Permissible) | PEL: 20 ppm |
| REL (Recommended) | 0.5 ppm |
| IDLH (Immediate danger) | 100 ppm |
| Related compounds | |
| Related compounds |
Morpholine N-Methylmorpholine 2-Ethylmorpholine 4-Ethylmorpholine Piperazine Diethanolamine |