Ethyl Piperidine-3-Carboxylate holds an interesting spot in medicinal chemistry. Its story begins in the labs of the mid-20th century, a time when researchers chased new heterocyclic structures to broaden their toolbox for drug development. Interest ramped up as the medical community’s focus turned to molecules that could structurally mimic neurotransmitters and other bioactive compounds. This ester derivative, built on the backbone of a piperidine ring, appealed to scientists working on synthetic routes for anesthetics, antipsychotics, and other classes of pharmaceuticals. Over the decades, organic chemists gradually refined its synthesis, keyed into scalable methods, and introduced variations of functional groups. With each improvement, the compound’s commercial and academic footprint increased—from niche laboratory curiosity to a standard building block in research worldwide.
The core structure of Ethyl Piperidine-3-Carboxylate makes it more than a passing interest in industrial and academic labs. The piperidine ring provides a strong starting scaffold, and the carboxylate ester group sets the stage for functionalization. I’ve encountered suppliers marketing this compound in various grades, from research-only to higher purity selections for active pharmaceutical ingredient synthesis. The physical product typically appears as a colorless to pale yellow liquid, stored in airtight containers to prevent moisture ingress and maintain stability. Most suppliers label it with CAS number 1126-09-6, using alternate names like 1-Ethoxycarbonylpiperidine or simply EPC.
Looking at the numbers, Ethyl Piperidine-3-Carboxylate has a molecular formula of C8H15NO2 with a molar mass sitting at 157.21 g/mol. Boiling point falls within 215-220°C, and a density near 1.02 g/cm³ at room temperature. The ester moiety, not surprisingly, imparts a characteristic sweet odor, but that is mainly relevant when handling in close quarters. It dissolves fairly well in organic solvents like ethanol, chloroform, and ether, but shows much less affinity for water—typical behavior for small esters. I’ve seen it crystallize under reduced temperature, but under standard storage, it remains a liquid, especially in unopened containers. This profile fits right in for folks interested in straightforward purification or downstream modifications.
Suppliers handling Ethyl Piperidine-3-Carboxylate document technical specifications with some rigor. Purity routinely reaches 98% or higher, with residual water less than 0.1% as measured by Karl Fischer titration. Heavy metals are almost always controlled below 10 ppm, given regulatory and safety pressures. Labels on bottles specify purity levels, the batch or lot number, recommended storage conditions (2–8°C, airtight, away from light), and the International Union of Pure and Applied Chemistry (IUPAC) name for clarity. Any hazards—flammable liquid, risk of skin/eye irritation—land right on the front, reinforced by GHS pictograms and hazard statements.
Anyone synthesizing Ethyl Piperidine-3-Carboxylate tends to start with 3-piperidinecarboxylic acid. The classic method involves Fischer esterification—combining the acid with ethanol and a catalytic drop of sulfuric acid under reflux. The reaction mixture heats until equilibrium is reached; at this point, excess ethanol helps drive the reaction to completion. I’ve also seen alternative routes using coupling reagents like DCC (N,N'-Dicyclohexylcarbodiimide), especially in scenarios where milder conditions are needed. Once the reaction finishes, extraction with nonpolar solvents like diethyl ether pulls the ester out of the aqueous mix before the final purification steps—commonly rotary evaporation and flash column chromatography to ensure high chemical purity.
Research chemists use Ethyl Piperidine-3-Carboxylate as a stepping stone for making a range of analogues. The ester group stands out for potential hydrolysis—using acid or base, one can revert back to the carboxylic acid or move to an amide by further reactions with amines. Reductive amination, alkylation, and even substitution on the piperidine nitrogen open up libraries of derivatives. Cross-coupling reactions further extend its significance in medicinal chemistry, especially for tuning solubility and bioactivity. Some routes produce arylated derivatives for CNS-active molecules, while others focus on incorporating fluorine or other small moieties to boost metabolic stability. Each transformation leans on robust literature methods, allowing teams to quickly probe structure-activity relationships in drug candidates.
The chemical market recognizes Ethyl Piperidine-3-Carboxylate under several tags. While its IUPAC name, ethyl piperidine-3-carboxylate, gets formal use, you’ll also spot commercial catalogs listing Piperidine-3-carboxylic acid ethyl ester, EPC, or its abbreviated chemical formula. CAS 1126-09-6 reliably connects buyers and sellers, even across different languages and regulatory frameworks. Researchers sometimes abbreviate names on internal paperwork, so vigilance with cross-checking structures, suppliers, and lot purity remains essential.
Handling this ester calls for careful attention to standard laboratory safety. Even though it doesn’t top toxicity charts, its volatility raises the risk of inhalation or skin exposure during sample transfer. Goggles, gloves, and lab coats make up the standard attire. Good air flow or use of a fume hood helps control odor and reduce inhalation risks. Safety Data Sheets, updated with the latest hazard information, instruct users on spill management, accidental exposure treatment, and proper disposal routes—usually incineration under controlled conditions. Every step tries to lower risk, especially during scale-up and transfer to manufacturing. Routine training reinforces best practices, and regular maintenance checks on storage setups—sealed glass containers, cool and dry conditions—cut the odds of unintended exposure.
Ethyl Piperidine-3-Carboxylate crops up often in pharmaceutical discovery, serving as a key intermediate for synthetic routes toward drugs acting on the central nervous system. Medicinal chemists rely on its framework to fashion molecules acting as antipsychotics, antidepressants, or anesthetics. Outside pharma, it appears in agrochemical research, offering a skeleton to build plant-protective agents. Academic labs also turn to it for structure-activity relationship studies, probing how tweaks to the piperidine ring impact biological activity. I’ve seen research into the environmental breakdown paths of its derivatives, looking for safer, slower-to-accumulate molecules for both medicine and agriculture.
The R&D space around Ethyl Piperidine-3-Carboxylate continues to grow. Teams explore its substitutions at each ring position, scanning for bioactive profiles that steer clear of unwanted off-target actions. Collaboration across chemistry, biology, and computational modeling accelerates new molecule design—moving faster from benchtop to animal testing and, ultimately, human studies. Patent activity reveals an uptick in CNS-active compounds—driven by unmet clinical needs around depression, schizophrenia, and neurodegenerative diseases. I see startups and universities leveraging advances in automated synthesis and real-time analysis, squeezing more information out of every batch. Custom analogues, built on this ester core, fill screening libraries for pharma giants, who hope the next blockbuster can start with a piperidine template.
Lab animal studies show that this compound falls into a moderate range of acute toxicity. Most incidents relate to inhalation or accidental ingestion, with outcomes tied to dose and duration. Repeat exposure can cause irritation in mucous membranes, and higher doses show impacts on the liver or nervous system. Occupational studies stress the importance of personal protective equipment and engineering controls in keeping incident rates low. Regulatory bodies update exposure limits based on fresh data, tightening workplace standards over time. Chronic toxicity hasn’t shown significant red flags in published reports, but as new analogues come into use, ongoing vigilance remains key to avoiding long-term health impacts.
Looking ahead, Ethyl Piperidine-3-Carboxylate will continue to carve out relevance in drug discovery. The piperidine scaffold, proved through decades of use, keeps showing promise as scientists screen for next-generation treatments. Advances in green chemistry will likely yield safer, less wasteful synthetic methods, while automation and AI-driven synthesis platforms may shrink development cycles. New toxicity screening platforms promise to accelerate preclinical evaluation, pushing safer and more selective molecules toward trial. Environmental stewardship sits higher on industry agendas—driving changes in waste management, greener reagents, and lower-emission work protocols. Every new study adds to its story, shaping where it fits in tomorrow’s pharmaceutical and chemical landscape.
Ethyl piperidine-3-carboxylate doesn’t turn many heads outside the world of chemistry or pharmaceuticals, but its role still matters for people depending on new medicines. Most laboratories see this compound as a building block—a foundation for making other, usually more complex, substances. Tracing its journey reveals how chemistry connects to daily life, even if you don’t see the links at first glance.
Many breakthroughs in modern medicine trace back to small molecules, and ethyl piperidine-3-carboxylate has served as a key stepping stone. Pharmaceutical chemists use it to create piperidine-containing molecules, a class trusted for decades as part of drugs for central nervous system conditions, anti-infectives, and heart disease. For years, companies have built antihistamines, antidepressants, and sedatives on similar frameworks. Its structure gives researchers control—they can swap out groups, test new arrangements, and move closer to a working medicine. I remember reading a research paper about antimalarial testing where changing just one link in the molecule made the difference between a strong drug candidate and a dud.
Organic synthesis pushes science forward. At universities and industry labs, researchers depend on reliable starting materials. Ethyl piperidine-3-carboxylate reacts smoothly with a range of other chemicals, making it a handy intermediate. It doesn’t sit on the shelf for long. Soon, it’s part of another batch—used to try out ideas, troubleshoot reactions, or design molecules with brand new shapes.
Drug pipelines need compounds that offer flexibility and solid chemical behavior, so ethyl piperidine-3-carboxylate remains a go-to choice. Some cancer research studies list it as a base for testing new inhibitors, especially where older approaches have hit a dead end. The hope is to uncover drugs that dodge resistance or work with fewer side effects. There are risks, too—every scaffold brings questions about toxicity, breakdown in the body, and cost to scale up manufacturing. Smart development always runs alongside careful checks for safety and sustainability.
People sometimes gloss over the controls around substances like this. Regulations and safety protocols matter as much as chemistry skills. Handling in the lab isn’t casual. Good ventilation, gloves, and careful labeling protect staff and students. At some facilities, locked cabinets keep intermediates like ethyl piperidine-3-carboxylate away from misuse. Strict reporting and tracking keep bad actors from repurposing these chemicals, since certain intermediates could show up in illegal drug production.
The conversation around chemicals often overlooks the people working with them every day. Our best action right now includes solid education for everyone handling new intermediates. Pharma leaders and professors I talk to agree—keeping a close eye on novel compounds, sharing data quickly, and upholding safety makes the entire field stronger. For outsiders, it’s enough to know that discovery and safety aren’t just word games—they show up whenever a new treatment reaches the world outside the lab.
Ethyl piperidine-3-carboxylate – this mouthful of a molecule pops up in research labs across pharmaceuticals and chemical synthesis. Folks tend to overlook the impact of tiny impurities in a bottle of chemical, but these traces can mean the difference between a successful experiment and a week thrown away. Purity grades create a layered landscape in the chemical supply world. I remember walking into the stockroom, hunting for reagents, and realizing two vials with the same name sometimes punch way above or below their weight depending on what you demand them to do.
Synthetic organic work can turn messy with the wrong grade. Researchers may chase 98%-plus purity for drug development or analytical use because even dust specks of impurity sneak into final data. Manufacturers label products with tags like “technical,” “laboratory,” or “analytical” grade. These aren’t marketing words; they describe actual refining processes. Technical grade may cut costs, but carries more byproducts. For industrial processes, say in polymer synthesis, a tiny bit of impurity hardly causes headaches. High-purity versions, though, sweep out nearly all byproducts, making sure nothing in the chemistry clouds crucial results or safety.
It’s easy to shrug off the difference between 95% and 99% purity. Once, during a routine reaction, a “pure enough” compound sabotaged sensitive equipment with trace metals. These experiences drive chemists to hunt down certificates of analysis. The stakes shoot up when a product walks into preclinical work or batch-scale pharmaceutical production. Unwanted trace chemicals don’t just mess with yields – they sometimes introduce unexpected toxicity or set off regulatory alarms. All of this pushes industry and researchers to ask, “just how clean is this bottle?”
Chemicals like ethyl piperidine-3-carboxylate run the gamut from technical (roughly 90-95% purity), through laboratory or research-grade (96-98%), and up to analytical grade (98-99.5% or better). Every notch upward means more raw material and energy poured into purification. Suppliers usually publish purity as a percentage on their product sheets, and major players – think Sigma-Aldrich, Alfa Aesar, TCI – show grades tailored for different uses.
Demand shapes what grades show up. Commodity chemicals, high-volume users, and some syntheses make do with broader cuts of purity. Research and pharmaceuticals chase the top end. Popularity matters, too. If a compound starts making waves as a synthetic intermediate, suppliers invest more in high-purity versions.
Picking the right grade involves checking what matters most – budget, performance, or safety. For a student running their first reaction, technical grade might do the trick. For clinical trial material, only top-grade passes the sniff test. Regulatory guidelines from agencies like the FDA tighten the leash on pharmaceutical quality. Quality control teams lean on analytical tools like HPLC and GC-MS to verify every lot, and suppliers have started sharing spectra and batch-specific certificates.
There’s still room for suppliers to demystify what each grade means and back it up with transparent documentation. Seeing impurities listed plainly in certificates turns what used to be a leap of faith into an informed decision.
Experienced chemists know – always read the fine print, talk to suppliers, and keep safety front of mind. Don't assume two bottles of the same label will give identical results. Open channels with vendors about structural or trace impurities, especially if reproducibility or compliance is on the line.
Chemicals might carry seemingly simple instructions, but even small lapses in storage can turn costly or flat-out dangerous. Ethyl Piperidine-3-Carboxylate is an organic compound researchers and manufacturers handle in labs globally, and safe storage keeps both people and data protected. I once worked in a lab where missing a single cap warning label nearly led to a whole batch getting contaminated. No matter how familiar a name sounds, giving proper respect to even routine storage steps makes all the difference.
Ethyl Piperidine-3-Carboxylate benefits from cool, dry conditions. Tucking it away below 25°C prevents unexpected chemical changes. Moisture seeping in from a humid environment can speed up hydrolysis, so always keep containers tightly sealed. A dry, well-ventilated room gives reliability, and regular checks on humidity levels offer extra peace of mind. Labs with busy workflows do well to station chemical hygrometers in storage spaces; small investments here nip big problems in the bud.
Direct sunlight often degrades organic compounds, and this one’s no exception. Store it in an opaque or amber container—some folks might think a shelf’s enough, but I learned the hard way after seeing a batch lose potency after weeks near a sunny window. A dedicated flammable storage cabinet meets both safety codes and keeps bottles out of high-traffic spots. It’s honestly easier to build safe routines than to clean up after a slip-up.
Glass vials or specialized polyethylene bottles resist chemical reactions and won’t introduce new hazards. Make sure each bottle has a clear, chemical-resistant label with the name, date received, and any hazard warnings. Even if you feel confident, labels prevent mix-ups and keep everyone on the same page—especially in shared spaces.
Regular audits help identify evaporation or unexpected changes. If the substance shows color shifts or forms odd residues, disposal is best. Those little changes tend to signal bigger trouble than most realize. I always recommend keeping only the quantities needed for 6 to 12 months; standing inventory starts to grow risk, not just dust.
Separation from oxidizing agents or acids reduces the chance of dangerous reactions. Never store ethyl piperidine-3-carboxylate near nitric acid, strong mineral acids, or open flames. I once made the mistake of stacking unrelated organics in one cabinet, and a single leaking cap led to a headache for the entire team. A few extra minutes reading Safety Data Sheets for every incoming shipment saves hours managing emergencies.
Spill kits, gloves, and eye wash stations near storage areas build confidence into the workflow. Equipping every user with the right safety training doesn't just tick compliance boxes—it shapes a culture where mistakes become less frequent. No one expects accidents until they’re mopping up the consequences.
Safe storage goes beyond technical instructions. It invites everyone working with chemicals to stay vigilant, revisit safety standards often, and never assume shortcuts save time in the long run. Whether running a university lab or a small start-up, smart storage keeps operations smooth and risk far from the door. With easy habits—cool temps, locked doors, and airtight labeling—labs protect people, results, and reputations every single day.
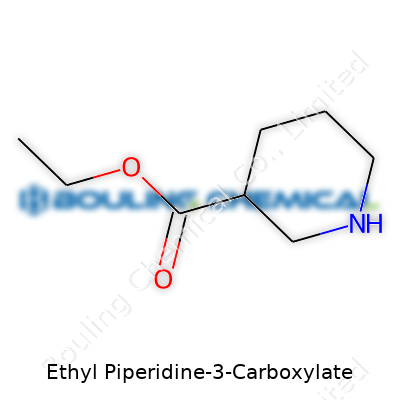
Ethyl Piperidine-3-Carboxylate carries the molecular formula C8H15NO2. Each part of this formula offers a clue about the chemical’s make-up: eight carbons, fifteen hydrogens, a nitrogen, and two oxygens. For anyone who’s cracked open an organic chemistry textbook or worked in a lab, that combination suggests a ring structure—here, a piperidine ring—with an ethyl ester tail. Synthetic chemists know this structure well, especially when they’re looking to tweak molecules for pharmaceuticals, flavors, or specialty catalysts. Once I needed to identify a precursor in a project screening antipsychotic candidates, and recognizing that unique carboxylate helped me pinpoint the right branch in a clutter of similar compounds.
The CAS number for Ethyl Piperidine-3-Carboxylate is 34841-35-5. In research and industry, CAS numbers often matter even more than a name. They give clarity in a world where molecular aliases can pile up—meaning a purchase order, a safety sheet, and an academic paper all connect clearly, without translation errors. I’ve seen teams waste days due to mixups caused by common names, especially as databases grow. The CAS number cuts through the confusion, letting sourcing teams and researchers know they’re truly talking about the identical substance.
Ethyl Piperidine-3-Carboxylate isn’t a household word, but it’s a familiar face behind the scenes in labs. Its ring system lends itself to building bigger molecules—especially where nitrogen rings add biological activity. Drug discovery programs love this backbone, plugging it into new potential antivirals, antipsychotics, and cardiovascular treatments. And in synthetic chemistry, it works as a cornerstone for making more advanced building blocks. I remember a synthesis campaign where we tried dozens of piperidine derivatives, looking for traits that let a candidate break through cell membranes efficiently, and this ethyl carboxylate version stood out for purity and yield.
Working with molecules like Ethyl Piperidine-3-Carboxylate brings real concerns for safe handling. With volatile organics, even a spill or splash creates exposure risks and clean-up headaches. Small companies and university labs often lack custom containment tools, so strong protocols and thorough training remain key. Sometimes, I’ve felt first-hand how a quick safety shortcut—say, a missing glove—can lead to skin irritation or worse. The compound also demands proper storage, since it reacts if left in the open with air or light.
For every challenge, better sourcing and training help. Suppliers now offer complete documentation, purity certificates, and clear hazard information, narrowing the risk of receiving something contaminated or mislabeled. Digital tracking using CAS numbers keeps inventories tight, so old reagents don’t linger forgotten on shelves. For safe handling, labs benefit from routine drills and visible checklists, reinforcing good habits in groups large and small. Ensuring that everyone who works with compounds like Ethyl Piperidine-3-Carboxylate knows its hazards—and its real value—makes progress possible without accidents or costly errors.
Ethyl piperidine-3-carboxylate looks like just another organic compound on a chemical inventory. To those who have handled a long list of lab reagents, it doesn’t sound more intimidating than a thousand other shelf dwellers. But spending time around chemicals has taught me to respect anything that worlds like pharmacology or agrochemicals touch. Even run-of-the-mill intermediates matter, especially if not enough people talk about their risks.
This compound often shows up as a building block in research, tucked away in syntheses for drugs or specialty products. The hazards aren’t always high-profile, but the specifics can make a big difference for graduate students, technicians, and chemists everywhere. Ethyl piperidine-3-carboxylate doesn’t carry the explosive reputation of some older intermediates, but its structure suggests caution. A piperidine ring combined with an ester usually signals mild to moderate risks, especially for skin, eyes, and the respiratory system.
I have learned—sometimes through small accidents—that skin and eye irritation are no joke. Many esters, including ethyl piperidine-3-carboxylate, may irritate on contact, and enough vapor in a small space can mean headaches or worse for a busy chemist. The literature flags acute risks related to exposure, and it always pays to check a fresh SDS even for chemicals you think you know. Though there’s no evidence this substance is outright toxic in tiny lab quantities, repeated or careless exposure adds up, especially during longer projects.
Most chemists I know pick up habits that matter more than official warnings. Glasses stay on, gloves are changed after every synthetic step, and lab coats usually keep the surprise burns and rashes at bay. Ethyl piperidine-3-carboxylate demands standard personal protective equipment: nitrile gloves, goggles, and fume hood work. No shortcuts here. Even minor splashes mean a trip to the eyewash or sink, and fume hoods offer more than just comfort—they lower vapor hazards and make spills easy to contain.
One slip-up I’ve seen too often is keeping volatile or reactive bottles near open work areas or heat sources. This ester type does not need refrigeration, but avoiding direct sunlight and storing away from strong acids or bases prevents slow breakdown and accidents. Shoot for well-labeled secondary containers, because confusion breeds errors. Ethyl piperidine-3-carboxylate does not belong in general waste or drains, and chemical disposal routes are usually spelled out for any organization with a compliance officer worth their salt.
No amount of warning signs replace a healthy respect for new chemicals. New students benefit most when a senior scientist walks them through the real-world quirks of compounds like this one. In my own lab, we share near-misses and lessons learned, fostering an environment where asking questions or updating labels gets more importance than bravado.
Solutions begin at the bench level. Checking the latest data sheets, storing containers properly, handling every reagent with the same care, and reporting all incidents quickly keeps accidents rare. No one regrets time spent learning how to deal with chemical risks—especially not the day small lapses become close calls.
| Names | |
| Preferred IUPAC name | Ethyl piperidine-3-carboxylate |
| Other names |
Ethyl 3-piperidinecarboxylate Ethyl 3-carboxypiperidine 3-Piperidinecarboxylic acid ethyl ester |
| Pronunciation | /ˈiːθɪl paɪˈpɛrɪdiːn θriː kɑːkˈsɒksɪleɪt/ |
| Identifiers | |
| CAS Number | 4549-42-4 |
| 3D model (JSmol) | `/polygon?modelid=3D/000/012/392/ethyl_piperidine-3-carboxylate&format=jsmol` |
| Beilstein Reference | 1710807 |
| ChEBI | CHEBI:85749 |
| ChEMBL | CHEMBL2116376 |
| ChemSpider | 24818684 |
| DrugBank | DB08378 |
| ECHA InfoCard | 18b71f72-e62a-4fb0-b473-9a8392a971ec |
| EC Number | 1045418-27-8 |
| Gmelin Reference | 92296 |
| KEGG | C19279 |
| MeSH | D008374 |
| PubChem CID | 11420086 |
| RTECS number | NJ3150000 |
| UNII | B3DF4G9Z2H |
| UN number | UN3271 |
| Properties | |
| Chemical formula | C8H15NO2 |
| Molar mass | 157.23 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | Characteristic |
| Density | 1.0 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 0.9 |
| Vapor pressure | 0.2 mmHg (25 °C) |
| Acidity (pKa) | pKa = 11.2 |
| Basicity (pKb) | 5.86 |
| Magnetic susceptibility (χ) | -61.7×10^-6 cm³/mol |
| Refractive index (nD) | 1.454 |
| Dipole moment | 3.49 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 217.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -4032.7 kJ/mol |
| Pharmacology | |
| ATC code | N06DX02 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin irritation, causes serious eye irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P272, P273, P280, P302+P352, P305+P351+P338, P312, P321, P332+P313, P333+P313, P337+P313, P362+P364 |
| NFPA 704 (fire diamond) | 1-3-0 |
| Flash point | 95.3 °C |
| Lethal dose or concentration | LD50 oral rat 1900 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 > 2000 mg/kg |
| NIOSH | Not established |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.7 mg/m³ |
| IDLH (Immediate danger) | No IDLH established. |
| Related compounds | |
| Related compounds |
Piperidine Piperidine-3-carboxylic acid Methyl piperidine-3-carboxylate Ethyl piperidine-4-carboxylate Ethyl nicotinate Ethyl isonipecotate |