People have been tinkering with piperidines since the early 20th century, mainly because the chemical skeleton offers versatility for fine-tuning properties in pharmaceuticals and other specialties. As organic chemists chased improved syntheses and cleaner reactions, ethyl piperidine-1-propionate started to find a role in labs that needed complex intermediates. In the 1980s, research around N-heterocyclic compounds picked up, and this led to renewed interest in piperidine derivatives, especially ones with ester functionalities. Universities and industry researchers pushed for better yields and purities, unlocking larger-scale production and more confident uses in fields like drug development and fragrance chemistry. For anyone who’s spent time in an organic lab, the iterative process that shaped this molecule’s production feels familiar—lots of trial, plenty of error, and eventual success driven by persistence instead of luck.
Ethyl piperidine-1-propionate shows up as a colorless to pale yellow liquid, sometimes with a faint, fruity odor if you manage to catch it before it’s diluted. The molecule combines a piperidine ring with a propionate ester, which gives it a unique combination of basicity from the nitrogen and lipophilicity from the ester side chain. Chemists grab this compound for its use as a building block, shifting between hydrophilic and hydrophobic domains in their target molecules. In my work, picking the right ester function helps design drugs that can either cross membranes more easily or stick around in the body for a tailored time, so a versatile molecule like this always attracts attention.
Looking closely at the numbers, ethyl piperidine-1-propionate usually sports a molecular weight around 185.28 g/mol. The boiling point sits high enough to hold up during most distillations but doesn’t require more brute force equipment unless you’re distilling at scale. Solubility trends show good mixing with organic solvents like ethanol or ether, thanks to the non-polar tail and the nitrogen enhancing miscibility in polar environments. The piperidine ring means moderate basicity and a tendency to participate in nucleophilic reactions. I remember trying to separate it from a similar amide using basic aqueous solutions—the amide washed away, the base held tight to the piperidine, and separation became a straightforward task.
Chemical suppliers usually sell ethyl piperidine-1-propionate with stated purity levels above 98%, ensured by gas chromatography. Labels stress low moisture content, as water encourages hydrolysis of the ester group, which can wreck intended uses or introduce byproducts. Standard containers use amber glass to shield from light and slow any unwanted reactions. The UN number and hazard codes line up with standard organic esters, flagging moderate flammability and mild toxicity. For researchers or operational staff, having transparent specs and batch documentation gives peace of mind and helps pinpoint any problems with synthesis or experimental outcomes.
Synthesizing ethyl piperidine-1-propionate boils down to a two-step protocol in most textbooks. Start with piperidine, then introduce 3-bromopropionic acid ethyl ester under basic conditions—usually triethylamine or sodium carbonate steps in as the base. The nucleophilic amine attacks the alkyl halide, forging a clean N-alkylation. Finish by washing with dilute acid, extract into ether or dichloromethane, and dry over magnesium sulfate. Crude yields already look decent, and with some distillation you can tighten up the purity. Any seasoned chemist has faced this type of process and knows that minor tweaks—reaction time, stirring rate, purity of starting materials—often determine whether the real-world result lives up to the literature.
Once in hand, ethyl piperidine-1-propionate opens doors for further chemistry. I’ve seen colleagues run hydrolysis on the ester to get the corresponding acid, which gives a handle for peptide coupling or other bio-relevant extensions. The piperidine ring’s nitrogen takes on functional groups in reductive amination, helping expand or customize the compound for specific targets. For those working with radiolabels or needing fluorescent tags, the nitrogen acts as a ready point for attaching additional markers. Some industrial teams use hydrogenation on similar molecules to tune properties without breaking the ester group, showing the kind of flexibility that keeps chemists coming back.
Chemical catalogs often list this compound under variations like "N-propionyl piperidine ethyl ester," "1-piperidine propionic acid ethyl ester," or simply "ethyl 3-(piperidin-1-yl)propanoate." Sometimes, suppliers use numbers tied to their own system or even patent-protected codes. Staying organized means tracking these alternate names, since missed synonyms can lead to ordering errors or misunderstandings in cross-team collaboration. Over time, seasoned lab managers keep a running tally to avoid costly mix-ups or delays.
Working with ethyl piperidine-1-propionate needs a good grasp on safety basics. The ester’s volatility means proper ventilation and fume hood setups are non-negotiable. Direct skin contact can lead to local irritation, and inhaling concentrated vapors triggers headaches or mild nausea—labmates who underestimate these risks learn fast after a spill or rough vapor exposure. Wrapping up containers tightly, labeling with both hazard symbols and concentration, and keeping spill kits on hand all rank as second nature to reduce accidents. In larger facilities, standard operating procedures stress double-checking bottle seals, segregating incompatible substances, and scheduling regular safety drills.
Ethyl piperidine-1-propionate wears a few different hats in modern chemistry. Pharmaceutical researchers look to its piperidine core for developing CNS-active drugs, capitalizing on this group’s track record in treating pain or psychiatric disorders. Its ester functionality allows controlled cleavage, making it valuable for timed or targeted drug release. The fragrance and flavors industry finds occasional utility in the molecule’s structure when building musky, persistent aromas, though safety profiles restrict widespread use. Some materials scientists weave it into specialized polymers or resins, searching for tuned flexibility or tailor-made responses to environmental triggers. In my experience, discussing real-world applications with scientists outside organic chemistry always led to new perspectives—cross-pollination between pharma and polymer science has brought on unexpected breakthroughs more than once.
Today, research teams continue to tinker with ethyl piperidine-1-propionate as a scaffold. Teams in drug discovery focus on the molecule’s role as a lead structure, especially in neuropharmacology, looking for new interactions with brain receptors. Computational chemists simulate variations, tweaking side chains to increase affinity or reduce unwanted effects. The academic community often pushes new synthetic pathways, aiming for greener approaches and cost reductions, such as flow chemistry or enzyme-catalyzed methods. Big pharma tracks patent trends, hungry for unique modifications that open new therapeutic areas or extend product lifecycles. Based on years in development labs, real progress usually follows honest collaboration and long hours spent troubleshooting side reactions or developing robust purification schemes.
Most available studies point to mild acute toxicity, though repeated high-dose exposure brings risk of liver and kidney stress. The ester group breaks down in vivo to release ethanol and propionic acid derivatives, both of which require monitoring due to potential metabolic disturbances. Animal trials often show reversible symptoms at moderate concentrations, but occupational guidelines stop short of recommending routine exposure without protections. Researchers keep pushing for more data—especially long-term toxicity and carcinogenicity studies, since new applications are under exploration every year. Many safety teams lean on updated MSDS sheets and real-life incident reports rather than pure academic literature, knowing that surprises can pop up once new usage patterns emerge.
Looking forward, ethyl piperidine-1-propionate sits in a good spot for expansion. As green chemistry trends pick up steam, companies look for esters that can biodegrade efficiently or work as intermediates in less wasteful syntheses. Advanced drug delivery, especially for slow-release or time-specific agents, may lean more heavily on this molecule as new linking strategies get refined. Cosmetic and flavor industries continue to scout for safer, more stable sensory compounds, and molecules with dual piperidine and ester structures remain of interest. As technology crosses into AI-driven compound libraries and high-throughput screening, structures like ethyl piperidine-1-propionate often get flagged for further examination. Based on my own experience, the compound’s versatility and manageable safety profile should keep it relevant in both established and emerging fields for years to come.
Ethyl Piperidine-1-Propionate doesn’t exactly roll off the tongue, but it pops up in some behind-the-scenes places. I’ve worked with chemicals for years and have seen the way compounds like this one slip quietly into products or research settings. Unlike some headline-making substances, Ethyl Piperidine-1-Propionate keeps a quieter profile, yet it has value in a few specific spots — particularly in fine chemical synthesis and pharmaceutical research.
This compound sometimes finds its way into the world of flavors and fragrances. Certain piperidine derivatives help form the backbone of flavoring agents or perfume elements, offering a base note that blends well. Companies aiming to replicate specific taste or aroma profiles investigate molecules like this to deliver a consistent scent or taste experience, especially when other ingredients are sensitive to heat or storage. I remember touring a fragrance lab and watching teams hunt for longevitiy and complexity in scents, creating molecules like Ethyl Piperidine-1-Propionate for testing in both food-safe and cosmetic blends.
Researchers rely on piperidine derivatives for their versatile chemical structure. Ethyl Piperidine-1-Propionate serves as a handy building block during drug development, helping chemists design molecules with the right shape and reactivity. For example, in early-stage research on treatments for neurological dysfunctions, piperidine-based compounds can be tweaked to alter how they interact with biological systems. One study tracked new molecules for possible use in treating Alzheimer’s symptoms, with modifications starting from the piperidine ring. That reflects a broader pattern: basic compounds, like this one, allow scientists to test hundreds of new options by tweaking side chains or introducing new groups.
Custom synthesis labs frequently use Ethyl Piperidine-1-Propionate as an intermediate. The goal often centers around making compounds that will never end up sold directly, but instead, end up one or two steps upstream from a final medication or specialty material. I’ve seen chemists map out long routes to their targets, where each step matters—not just for cost, but for safety and purity. Managing the handling of intermediate chemicals remains a challenge; guidelines for safe storage and transportation are strictly enforced. The point here is that building blocks like Ethyl Piperidine-1-Propionate set the stage for further innovation, even if most people never hear their name.
No one wants to discover that a broadly used chemical turns out risky for worker health or for the environment. For example, skin and respiratory irritation can crop up if labs or factories don’t use proper ventilation or basic protective gear. Regulatory bodies take a close look at documentation, insisting that all handlers understand potential dangers and have genuine access to gloves and fume hoods. Mistakes happen when training or communication falters, so ongoing education on chemicals like Ethyl Piperidine-1-Propionate protects everyone in the process—from warehouse staff to researchers at the bench.
It’s worth mentioning that accountability matters as much as innovation. Cleaner alternatives and careful record-keeping help the industry track where compounds go and how they’re handled. Digital tools that flag hazardous routes and improper storage can catch problems before they reach people or products. Open communication among labs, regulatory groups, and even the public encourages trust. As research and product development speed up, it’s on everyone in the field to balance curiosity with caution—and to make sure that chemicals like Ethyl Piperidine-1-Propionate leave a positive mark, not an unintended mess.
Ethyl Piperidine-1-Propionate is a mouthful for sure, but its structure tells a pretty interesting story if you break it down. You’ve got a molecule based on piperidine, a ring of five carbon atoms and one nitrogen atom, much like the backbone found in several pharmaceuticals and flavor compounds. Attach a three-carbon propionate group to that nitrogen, then tack on an ethyl group as an ester, and you’ve got this compound. Chemists usually write its structure as C5H10N(C2H5O2C)(C3H7)—but for most folks, the important thing is what all that means in real life.
I’ve seen a lot of buzz about new molecules, mostly because they drive innovation in fields like pharmaceuticals, flavors, or advanced materials. With a structure like Ethyl Piperidine-1-Propionate, you’re looking at a molecule that can blend properties. Piperidine rings show up everywhere, from drugs that help with neurological conditions to artificial flavors found in food. By linking in a propionate ester, you create a compound that can move through fats and oils more easily than a straight-up amine. Its shape can affect how it interacts with enzymes or smells to the nose.
Structure guides function in chemistry. The piperidine ring gives stability and a certain flexibility, letting this molecule wiggle through both water-based and oil-based mixtures. The propionate part carries the potential to act as a metabolic signal or be broken down by specific enzymes in the body. And that ethyl group hanging off the end can decide how quickly the body uses up the compound.
You won’t find Ethyl Piperidine-1-Propionate sitting on every lab shelf, but compounds in this family show up in drug design. Piperidine rings anchor some painkillers, antipsychotic drugs, and anti-tuberculosis agents. Small changes on this base structure can shift how these compounds act — a lesson I learned while working alongside medicinal chemists trying to tweak drugs for better results. Swapping out a methyl group for an ethyl or attaching different acid chains leads to new properties, possibly unlocking a more effective treatment or a better flavor enhancer.
On the industrial side, modifying chemical rings like this can improve how easily a flavor dissolves in oil or water. Flavors with piperidine backbones often end up in snacks and beverages, where their ring gives a peppery, grassy, or savory kick. With a propionate ester attached, the profile shifts yet again, perhaps making the compound more suitable for baked goods or beverages.
Safety stands front and center anytime new molecules hit the scene. Chemists put these new compounds through a series of tests for toxicity, allergenicity, or even environmental impact. You don’t want something that smells great but lingers in soil or water for years without breaking down. Regulatory agencies require full breakdowns, not just of the compound itself, but also how it falls apart in the body and in the wild.
The path from discovering a new chemical structure to putting it to work takes real teamwork across research, regulation, and industry. Chemists keep exploring how tweaks to piperidine-based rings can lead to better medicine and safer additives. Open communication with public health professionals and regulators paves the way for smart decisions about safety and benefit. It’s all about building confidence in these molecules, not only in the lab but in every place these compounds make a difference.
Anyone who’s worked at a lab bench knows chemicals have personalities. Some demand special gloves, others need ventilated hoods, and every so often, one arrives whose label sends you searching for the SDS before you even open the bottle. Ethyl Piperidine-1-Propionate sits in that category. If a material isn’t familiar, caution should guide every move.
This compound, as with many specialized reagents, doesn’t have broad household use. Researchers typically encounter it in synthesis projects or pharmaceutical development. Just because it’s not a household name doesn’t make it harmless. Chemists recognize that structural features count. The piperidine ring signals possible irritant properties, while the ester side often brings unpredictable volatility. That combination prompts respect.
In practice, safety starts before anyone touches the reagent. An up-to-date Safety Data Sheet (SDS) forms the essential foundation for risk management. Unlike vague safety posters, the SDS provides substance-specific data, pulled together by scientists who’ve documented firsthand incidents, published toxicological data, and controlled lab spills. According to published toxicology databases and supplier information, inhalation, skin contact, or accidental ingestion all pose real risks for ethyl piperidine-1-propionate: respiratory irritation, skin burns, and eye damage sit at the top of concerns.
Researchers who survive long careers don’t rely on hope or luck. Proper PPE matters—a fitted lab coat, nitrile or better gloves, splash goggles, and closed shoes create a protective layer. Working inside a chemical fume hood protects from vapors that may escape, and it also makes it less likely for unexpected splashes to reach the eyes or skin. A little preparation spares a lot of regret.
Safety procedures act as one part of the puzzle. Equally important, though, is real-world training. New team members must actually see demonstrations—reading a checklist doesn’t replace watching someone handle a volatile bottle safely. Hands-on training, led by workers with years of muscle memory, covers the gap between theory and reality. Everyone in the lab benefits when one mistake gets turned into a group lesson.
Accidents don’t start out as disasters. A spill contained in a fume hood, cleaned by someone who knows the protocol, becomes a minor issue. No chemical, no matter how familiar, lets those who cut corners off easy. In the event of a splash, emergency eyewash stations and showers can’t be afterthoughts. They need to be within fifteen seconds’ reach—that’s not just a guideline, it’s a number that’s been earned over decades of hard-learned lessons.
Labs can’t rely only on personal caution. Institutional controls offer a bigger safety net: clear chemical storage policies, good ventilation, and periodic safety audits stop problems before they become emergencies. Everyone—from the principal investigator to the undergraduate assistant—bears a share of responsibility. In my career, safe habits developed during mundane experiments have prevented problems whenever something unexpected happened.
Sometimes projects can swap more hazardous compounds for safer alternatives. Green chemistry advances show that, with creativity, scientists can reach their goals without unnecessary risk. But no shortcut makes up for cutting fundamental safety steps. Facing a chemical with real risks, respect serves as the best defense. If in doubt, ask a colleague who’s seen more years at the bench—the wisdom of experience rarely misses the mark.
Anyone who’s spent time working with specialty chemicals knows that safety starts with knowing your chemicals inside and out. Ethyl Piperidine-1-Propionate rarely features on household shelves, but in chemical research and manufacturing, it’s a familiar substance. The risk hides in plain sight: it’s both a liquid and volatile, known for its role as an intermediate in synthesis work, especially pharmaceuticals or advanced materials.
Daily practice in a lab shows that easy mistakes can create big headlines. This liquid reacts with moisture, and exposure to air can slowly compromise its quality—not to mention health and safety for anyone nearby. Glass containers with thick, tight caps stand up best over weeks or months. Polyethylene bottles also work, but glass doesn’t leach, and it’s easy to spot any changes in the solution.
Darkness keeps reactions slow. Most bottles on my shelf sit behind UV-blocking glass or in a metal cabinet, away from windows and lights. That’s not just overkill—it reduces the risk of breakdown and random sparks. Once, I saw a clear bottle left out under fluorescent lighting. The contents changed color, and it turned up as contamination during later tests. Nobody forgot the lesson.
Temperature stands out as a silent threat. Ethyl Piperidine-1-Propionate prefers it cool—room temperature in most climates, but on hot days, a dedicated flammable chemicals fridge keeps things stable. I always check the thermometer before locking up for the day. Closer to body temperature, the vapor gets much more noticeable. High temps slacken the cap seal and create leaks, so cool storage solves multiple problems at once.
Even trace moisture from the atmosphere leads to minor hydrolysis over time, producing unwanted byproducts. In the lab, I always dry bottles before filling them and dash a bit of desiccant into the storage area, not in the bottle itself. A simple silica gel pack lies hidden on nearly every shelf. Labels with names, dates, and concentrations make it easy to spot a problem and avoid mix-ups.
Never store acids or oxidizers within arm’s reach. I recall working with a benchmate who accidentally racked a corrosive cleaner beside our ethyl piperidine mix. The smallest spill or leaky cap could have forced an evacuation. Lab safety comes from paying attention to these pairings, and written inventories stop these mistakes from happening repeatedly.
Expiry dates matter. A manager once set up a “first in, first out” system. Oldest bottles in front, fresh stock in back. Immediately, we saw less waste and never had to question stability. A chemical like Ethyl Piperidine-1-Propionate loses performance as it ages—not to mention safety. Regular checks and updated logs catch problems before they hit the workbench.
No magic trick keeps chemicals safe—just careful attention, clear labeling, and respect for their quirks. Ventilated cabinets, simple organization, and good communication put the odds on your side. If you don’t have written storage protocols, now’s the time to create them. The effort pays off by preventing accidents and preserving purity. Each day without a mishap tells you the system works.
Someone inquiring about Ethyl Piperidine-1-Propionate wants more than a bottle of simple solvent. This chemical attracts attention for its use in research, pharmaceuticals, and specialty synthesis. I’ve watched chemical supply chains shift from open to closely watched spaces, especially around substances with possible dual uses or regulatory scrutiny.
Ethyl Piperidine-1-Propionate isn’t a compound you’ll find on supermarket shelves or in artisanal apothecaries. Chemical suppliers that serve research organizations or universities are the main sources. These companies—like Sigma-Aldrich, TCI Chemicals, or Alfa Aesar—usually sell to vetted institutional buyers. In the past, I’ve seen even academic orders for specific research chemicals stall out because a single compliance officer flagged a compound. The rise in stricter European and US chemical regulations has only thickened the paperwork and narrowed direct consumer availability.
There’s real logic behind the gatekeeping. Chemical safety and anti-diversion rules flow from hard lessons learned from accidents and illicit labs. A company like Sigma-Aldrich follows a strict buyer verification process for anything even vaguely categorized under "specialty chemicals." I can’t recall a time when a university researcher sailed through their ordering page without paperwork, letters of intent, and plenty of patience.
Governments watch for anything misused in clandestine drug synthesis or dangerous experiments. Local authorities in the US look to the DEA and state boards, while Europe leans on REACH and local equivalents. These rules affect how much you can order, why you want it, where it ships, and who can touch it. Safety and transparency keep tragedies and scandals at bay, but they frustrate those with honest needs.
Researchers and companies often feel stuck between the need for specific chemicals and a daunting maze of regulations. Orders get rejected or delayed for missing documentation. The focus on “know your customer” checks trips up small biotech startups or labs without established supplier relationships. I’ve heard many complaints from grad students chasing critical reagents, only to get caught in compliance limbo for weeks.
Tackling gatekeeping requires more than hoping for lenient rules. Building rapport with licensed distributors remains the best shot. Professional buyers should prepare proper intent letters, safety plans, and authorization documents. Long-standing relationships with reliable suppliers make a difference, as does joining recognized research consortia.
On a broader scale, industry groups and universities could lobby for streamlined processes for low-risk, research-scale purchases. Group purchasing or registries may help establish trusted identities, bringing together buyers and sellers in a transparent, auditable framework. Education—both for suppliers and customers—shrinks misunderstandings and delays.
Ethyl Piperidine-1-Propionate doesn’t present unique difficulties beyond the reality of buying specialty chemicals in today’s world. Focusing on safety and compliance, while smoothing legitimate access, holds the key. Responsible supply and use depend on people who study the rules and work with, not around, the system.
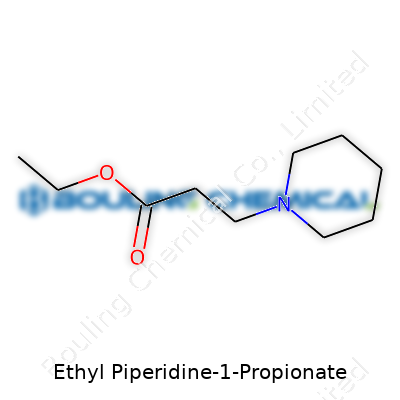
| Names | |
| Preferred IUPAC name | Ethyl 3-(piperidin-1-yl)propanoate |
| Other names |
Ethyl 3-(1-piperidinyl)propanoate Ethyl 1-piperidin-1-ylpropanoate Ethyl 3-piperidin-1-ylpropionate |
| Pronunciation | /ˈiːθɪl paɪˈpɛrɪdiːn wʌn prəˈpɒneɪt/ |
| Identifiers | |
| CAS Number | 4795-08-0 |
| 3D model (JSmol) | `JSmol.loadInline("C(COC(=O)CCN1CCCCC1)CC")` |
| Beilstein Reference | 1841837 |
| ChEBI | CHEBI:77956 |
| ChEMBL | CHEMBL4152964 |
| ChemSpider | 52453805 |
| DrugBank | DB08910 |
| ECHA InfoCard | ECHA InfoCard: 100_098_765 |
| EC Number | “629-801-7” |
| Gmelin Reference | 65328 |
| KEGG | C19103 |
| MeSH | D000082073 |
| PubChem CID | 126595815 |
| RTECS number | UF2975000 |
| UNII | GO33ZK5O8I |
| UN number | UN3272 |
| CompTox Dashboard (EPA) | DTXSID7026295 |
| Properties | |
| Chemical formula | C10H21NO2 |
| Molar mass | 185.29 g/mol |
| Appearance | Colorless to light yellow liquid |
| Odor | fruity |
| Density | 0.972 g/cm3 |
| Solubility in water | Slightly soluble in water |
| log P | 1.7 |
| Vapor pressure | 0.3 mmHg (25°C) |
| Acidity (pKa) | 10.98 |
| Basicity (pKb) | 5.68 |
| Magnetic susceptibility (χ) | -73.27e-6 cm³/mol |
| Refractive index (nD) | 1.445 |
| Viscosity | 30.1 mPa·s |
| Dipole moment | 4.02 Debye |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 417.6 J·mol⁻¹·K⁻¹ |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS05, GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P280, P303+P361+P353, P305+P351+P338, P370+P378 |
| NFPA 704 (fire diamond) | **1-3-1** |
| Flash point | 87 °C |
| Autoignition temperature | 220 °C |
| Lethal dose or concentration | LD50 oral rat 1514 mg/kg |
| LD50 (median dose) | LD50 (median dose) of Ethyl Piperidine-1-Propionate: 300 mg/kg (oral, rat) |
| NIOSH | NA8300000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Ethyl Piperidine-1-Propionate is not specifically established by OSHA. |
| REL (Recommended) | N/D |
| Related compounds | |
| Related compounds |
Piperidine Ethyl 3-piperidinecarboxylate 1-Propionylpiperidine N-Ethylpiperidine Piperidine-1-carboxylic acid 1-(3-Bromopropyl)piperidine Piperidine-1-propionic acid |