Chemists have been exploring the field of heterocyclic compounds for more than a century, always hunting for molecules that open new doors across pharmaceuticals and materials. The story of ethyl imidazole-4-carboxylate goes back to basic imidazole chemistry discovered in the 19th century. Early on, imidazole rings stood out for their presence in biomolecules—think histidine or biological cofactors—which led researchers to probe modifications like esterifying with an ethyl group at the fourth position. The work in these labs often involved tedious, time-consuming batch reactions and tricky purification steps. Over decades, as analytical techniques grew sharper and synthetic chemistry matured, pathways to ethyl imidazole-4-carboxylate became much more accessible and reproducible, fueling its adoption in academic and industrial research.
Among heterocyclic esters, ethyl imidazole-4-carboxylate finds its place as a key building block. Its value lies not just in the imidazole core but also in the reactivity delivered by the carboxylate side group and ethyl tail. Laboratories appreciate how it serves both as an intermediate for further transformations—such as amidation or reduction—and as a scaffold for drug discovery projects. The compound usually arrives from suppliers as a crystalline powder, white or off-white, and carries a sharp, sometimes pungent odor typical of imidazoles. Shelf life matches up with other low-molecular-weight esters, provided it is stored in a cool, dry place, sealed away from moisture or strong acids and bases.
Ethyl imidazole-4-carboxylate comes in with a molecular weight that sits comfortably within the sweet spot for early-stage synthetic intermediates. Its melting point ranges from about 80 to 84 degrees Celsius, signaling both purity and proper crystallization practices. Solubility in polar organic solvents makes it a flexible option during reaction planning—acetonitrile, DMSO, and methanol all work. The imidazole ring, fairly stable under neutral conditions, does not stand up well to long exposure to sunlight or oxidative agents, so light-proof storage matters. Chemical shifts on proton NMR are distinctive, which helps researchers verify identity and purity without wrestling each time with hard-to-read spectra.
Practical work in labs grows easier when technical details are clear. Reliable suppliers mark batches of ethyl imidazole-4-carboxylate with lot numbers, purity (often 98% or above), water content, and hazard labels for irritancy or inhalation risk. Container material matters: glass or high-density polyethylene tops the list for avoiding contaminant leaching. Every consignment includes an up-to-date Safety Data Sheet, revealing everything from melting point and boiling point to recommended PPE. Some manufacturers present spectral data—NMR, IR, and HPLC—granting peace of mind that standards align with target quality metrics and regulatory expectations.
Most batches derive from a condensation reaction that brings together glyoxal with ethyl carbamate, promoted under mildly basic conditions using a source of ammonia or ammonium salts. Another efficient approach uses direct esterification or Fisher-type chemistry, with imidazole-4-carboxylic acid activated by thionyl chloride or acid catalysts before reaction with ethanol. Lab-scale preparation focuses on minimizing side products: careful temperature control, high-purity reagents, and flash chromatography deliver the tightest purity. Environmental concerns spur development of greener methods as well, with many chemists now using solid-phase catalysts or less toxic solvents to cut down on waste and lower overall impact.
The reactivity of ethyl imidazole-4-carboxylate invites a host of chemists to tweak it for their own needs. The ethyl ester group can get swapped for other functional groups, or hydrolyzed down to the free acid, which opens up even more doors for coupling strategies. Direct N-alkylation at various ring positions tailors the backbone for different biological or materials applications. Suzuki or Heck coupling reactions installed at the imidazole ring lead to more complex molecules, while reduction—either using borane reagents or catalytic hydrogenation—generates alcohols or amines ready for deeper derivatization. This flexibility explains why the molecule often features in patents for new pharmaceuticals or functional polymers.
Researchers run into ethyl imidazole-4-carboxylate under different names, depending on supplier or region. Some catalogs call it ethyl 1H-imidazole-4-carboxylate, or simply imidazole-4-carboxylic acid ethyl ester. Other trade names use shorthand like EI4C or variants tied to IUPAC nomenclature. This confusion frustrates catalog searches but ensures cross-referencing remains critical for ordering, regulatory filings, and patent translations. Awareness of synonyms prevents costly ordering errors and regulatory snags during shipping or customs checks.
Working with small-molecule imidazoles means respecting their moderate risk profile. Gloves, goggles, and proper ventilation hold the line against skin or respiratory irritation. Spills call for immediate clean-up using absorbent pads, and waste goes into halogenated organic waste streams. Chronic inhalation risk grows without good fume hoods, and some individuals show skin sensitization. Researchers must review Safety Data Sheets thoroughly, not only for personal safety but also to comply with environmental regulations around disposal and reporting. Emergency wash stations and spill kits prevent accidents from escalating, especially in teaching labs or scale-up facilities.
Chemists reach for ethyl imidazole-4-carboxylate when developing new pharma compounds, often as a core intermediate for antifungals, antivirals, or enzyme inhibitors. It shows up in agricultural chemistry too, installed into larger molecules to tune solubility and target specificity. Polymer labs value the imidazole nucleus for ion conductors or poly-electrolyte membranes, and researchers working on sensors exploit its electron-rich character. Medicinal chemistry claims the broadest use; the structural motif often appears in screening libraries, feeding a cycle of hypothesis and experimental data that brings new therapies closer to reality. Some specialty chemical suppliers pitch it for use in dye or pigment synthesis, especially where stability across pH changes is essential.
Academic labs continue to probe new facets of ethyl imidazole-4-carboxylate, aiming for more efficient routes or new mechanisms of action. Drug discovery outfits design analogs based on the imidazole ring’s hydrogen-bonding capabilities. Material scientists build block copolymer chains with it, citing better ion mobility in battery membranes. Every year, new journal articles and patents emerge—often from groups pushing the molecule into surprising territory, such as enzyme mimetics or metal-organic frameworks. Funding agencies see value in supporting these projects, especially as wider sustainability and pharmaceutical access come into focus.
Researchers have put ethyl imidazole-4-carboxylate under the microscope to chase down any acute or chronic effects. Preliminary work in cell culture notes moderate cytotoxicity at high concentration, though far below dangerous industrial chemicals. Oral toxicity data in rodents trends toward low risk at exposure levels common in lab or pilot-scale usage. More studies focus on breakdown products—what happens if the molecule degrades inside the body, or during disposal. Most breakdown routes lead back to imidazole and carboxylic acid fragments with lower toxicity, though labs working at scale still favor proper personal protection and strict handling protocols. Ongoing toxicity research aims to cover gaps as new applications spread.
Across sectors, the future of ethyl imidazole-4-carboxylate shines brighter as researchers demand more customizable, stable intermediates. As green chemistry gains importance, production trends move away from harsh catalysts and volatile solvents, replacing them with renewable feedstocks and safer conditions. Advances in continuous flow chemistry could bring cost and efficiency gains, making it easier for small firms to join the supply chain. Pharmaceutical pipelines remain hungry for new leads, and the sheer chemical versatility of the imidazole core will keep this compound in the mix for years to come. Environmental testing and tightening safety guidelines will spark new rounds of research, driving better understanding and, ideally, safer, greener derivatives for the next phase of industrial chemistry.
Ethyl Imidazole-4-Carboxylate doesn’t usually make headlines, but its role in chemistry labs is nothing short of important. The compound, which shows up as a white to slightly off-white powder, forms the backbone of several crucial chemical processes. If you’ve ever tinkered with organic chemistry, you might remember the imidazole ring showing up as a key structure in enzyme research, medicine, and material science.
This compound goes to work as an intermediate in drug manufacturing. Put simply, it acts as a starting piece in crafting more complex medicines. Scientists rely on it for building antifungal agents, antimicrobial drugs, and sometimes even cancer therapy candidates. The imidazole ring structure, present in a lot of synthetic and natural drugs, often brings about good interactions with proteins in the body, lending value to Ethyl Imidazole-4-Carboxylate in the pharmaceutical world.
Several studies highlight the relationship between these imidazole-related compounds and antifungal activity. Fluconazole, for example, includes an imidazole core. Companies that produce building blocks like Ethyl Imidazole-4-Carboxylate give drug developers solid foundations upon which new treatments can emerge. Real-world impact follows: new antibiotics and cancer treatments often start from small, stable molecules like this one.
Imidazole derivatives do more than build medicines. Researchers also use Ethyl Imidazole-4-Carboxylate in synthesizing specialized molecules for fuels, sensors, and polymers. Some of these applications stretch straight into the electronics industry. OLED displays, for instance, have benefitted from derivatives built upon this compound. The presence of an ethyl ester group gives it flexibility to slot into a range of custom synthesis projects.
In my own handful of research projects at university, we used similar imidazole compounds in the search for new battery materials. These small molecules gave us several handles for controlling charge transport. Colleagues in biotechnology often reach for these intermediates while crafting probes to track cellular reactions, because the chemistry plays well with others, linking up neatly without introducing unwanted byproducts.
Chemicals like this one demand respect. Breathing dust from powders, getting it on skin, or mishandling waste can bring on issues ranging from mild irritation to larger safety risks. Researchers and production staff—myself included—always keep gloves and eye protection close at hand. Facilities with good ventilation and strict labeling prevent many accidents before they even happen. Responsible sourcing also matters. Working with trusted suppliers who prioritize quality, traceability, and environmental responsibility ticks all the right boxes.
The world faces constant pressure for cleaner, safer drugs and next-generation materials. The practical work often starts with lesser-known building blocks like Ethyl Imidazole-4-Carboxylate. Instead of searching for silver bullets, researchers and manufacturers spend thousands of hours experimenting with ingredients like this, troubleshooting reactions, and narrowing down the safest, most effective candidates for medicine or technology. The value lies in the details: chemical companies disclosing purity data, sharing safe-handling tips, and supporting supply chain transparency. Through collaboration, both science and society draw real benefit from such an unassuming compound.
Ethyl Imidazole-4-Carboxylate might never become a household name. Still, its usefulness in drug discovery, material science, and chemical manufacturing speaks volumes. By supporting research, transparent sourcing, and rigorous safety procedures, this compound and others like it quietly push science forward, making fresh treatments and technologies possible.
Ethyl imidazole-4-carboxylate looks simple on paper but tells a story at the molecular level. Its structure draws from the imidazole ring—five atoms, two of them nitrogen, joined in a flat, aromatic ring. At position four, a carboxyl group hangs on. That group finds itself turned into an ester with ethanol, hence the “ethyl” in the name. Standard chemical notation lists it as C6H8N2O2. The ring keeps things rigid while the ethyl ester adds flexibility and helps the molecule dissolve in more than just water.
In labs, formulas don’t sit on shelves for the sake of looking pretty. Ethyl imidazole-4-carboxylate shows up in pharmaceutical research, especially when chemists want to build more complicated molecules. That’s because the imidazole ring exists in a lot of drugs, ranging from antifungals to antihistamines. Literature points out that many kinases—the enzymes that run cell signaling—react with imidazole derivatives. Without some creativity in designing new molecules, a lot of those enzyme blockers wouldn’t exist.
Try producing certain drugs without an intermediate like ethyl imidazole-4-carboxylate and things get pretty complicated. Medicinal chemists count on single steps to put rings and substituents in the right place. If the structure changes even a little—say a methyl group drops in, or the ethyl group switches to something bigger—the final product acts differently. Even tiny tweaks in the structure shift the way the molecule fits into enzyme pockets. As someone with experience in analytical chemistry, seeing small changes create big results always feels a bit magical, but it’s pure logic and geometry in action. This compound is one of those building blocks that highlights the importance of precise structure.
Safety remains a real concern with a compound like this. Imidazole esters aren’t considered especially hazardous, but vigilance keeps everyone out of trouble. Direct skin contact doesn’t burn but repeated exposure creates headaches and possibly sensitization over time. Fact sheets from trusted chemical suppliers stress good ventilation and gloves—not a place to cut corners. The stakes grow higher in pharmaceutical plants where scale ramps up the risk, making proper handling and disposal a necessity rather than a formality.
Green chemistry continues pushing for ways to make precursors like ethyl imidazole-4-carboxylate without harsh reagents or waste. Researchers experiment with enzymes as catalysts instead of strong acids or bases. Those alternatives avoid dangerous byproducts and result in less polluted water downstream. Solvent choice also matters. Swapping out older organic solvents for bio-based or recyclable options keeps environmental impact lower, a point that regulators and the public care about more every year.
Recognizing the value of a core building block like ethyl imidazole-4-carboxylate helps bring more thoughtful decisions to drug development and lab practice. Awareness of structure, handling, and environmental impact raises the standard for both experienced chemists and those just starting in the field. This mindset change gets results—better safety records, more effective drugs, and cleaner chemistry. The conversation around chemicals like this won’t end here, and that’s a positive shift for science and for society.
Anyone who’s spent time around a lab knows storage isn’t just about finding shelf space. Ethyl Imidazole-4-Carboxylate, with its delicate structure, asks for a thoughtful approach. Its integrity can drop fast if left in the wrong spot or mixed with the wrong substances. While the molecule isn’t known to explode at room temperature, chemists grow up respecting how quickly chemicals can react if overlooked. Accidents rarely come with warning labels.
This compound handles best below 25°C, a lesson that shows up all over chemical storage guides. My early days in research taught me that ‘cool, dry, and well-ventilated’ reads simple on paper but keeps more than just the molecule safe. Labs often use storage cabinets in climate-controlled rooms. Direct sunlight climbs the risk meter quickly—light can sneak in, break bonds, and degrade the compound long before someone notices. Extra heat from nearby machines can have a similar effect, even in a so-called ‘controlled’ room.
You might brush off moisture as a minor nuisance, but humidity can ruin plenty of substances without anyone seeing the damage happen. Silica gel packets or other desiccants earn their place in most chemical cabinets because they suck up stray water in the air. Ethyl Imidazole-4-Carboxylate loves staying dry. Once moisture creeps in, you face the possibility of hydrolysis or unwanted clumping. I’ve seen bottles ruined by careless re-capping or storing in places where condensation forms—sometimes in as little as a weekend.
Stacking chemicals alphabetically sounds tempting. It’s also a shortcut to trouble. Ethyl Imidazole-4-Carboxylate stays most stable in isolation from acids, bases, and strong oxidizers. Cross-contamination happens faster in busy labs—errant fumes, residue on gloves, or splashes from neighboring bottles. Separate shelves, clear labels, and never reusing an old bottle for a new chemical can dodge headaches and paperwork.
Glass usually wins the day for organic molecules. Using plastics sometimes invites unexpected chemical reactions or leaching, especially across long storage periods. Tightly sealed caps help more than most realize. Loose lids give moisture and oxygen a free pass, risking slow breakdown that erases the reliability of results. Amber bottles add extra protection from stray UV light, a tip passed down from anyone who’s opened a ruined reagent.
I’ve worked in spaces where half the risk wasn’t from explosions, but from forgetting what lurked in the back of the cabinet. Labeled dates, lot numbers, and hazard categories aren’t just for compliance—they help trace every reagent’s journey. Most labs run periodic checks. If something sits too long, responsible disposal trumps wishful thinking. Using older stock first, rotating inventory, and double-checking before a project begins stops surprises down the line.
It’s easy to glance at old containers or familiar compounds and forget why storage rules matter. I always tell new lab members: conditions don’t just protect the molecule—they protect everyone in the room. At the end of a project or a crazy-long day, making the right call about where and how to store something makes science safer for the next in line.
People who work in pharma labs and chemical manufacturing have likely come across Ethyl Imidazole-4-Carboxylate, known to many as an essential intermediate. The main reason demand rises boils down to its role in crafting advanced molecules, those often needed for drug discovery or specialty materials. Its chemical backbone brings flexibility and opportunity for synthesis teams hunting for routes that cut waste or shorten multi-step reactions.
Sourcing a kilogram or two isn't difficult. Plenty of catalog suppliers list Ethyl Imidazole-4-Carboxylate for research quantities. The story gets more complicated when somebody needs ten kilos or a barrel’s worth. Chemical catalogs stall out at small sizes, and global supply chains don’t always spotlight this specific cas number. Only a handful of specialized suppliers handle such custom bulk orders, and their lead times fluctuate according to raw material flows, synthesis routes, and regulatory backgrounds.
As a bench chemist who’s tracked down rare heterocycles, I’ve learned to contact suppliers directly and negotiate on purity, packaging, and documentation. It helps to have a clear technical sheet and to know the minimum order quantity that actually kicks off a batch. Most suppliers prioritize their standard products, so non-standard items like Ethyl Imidazole-4-Carboxylate need a firm commitment from buyers ready to back their request with volume, not just a quote.
Sourcing in bulk means more than just volume; it brings the question of traceability and compliance. Within Europe, REACH registration affects what can legally cross borders or be used at scale, while in the US, suppliers stick tightly to EPA and FDA guidance for intermediates. Some countries do not list this chemical on their controlled substances schedules, letting producers operate with less oversight, but that doesn’t guarantee safety, consistency, or regulatory peace of mind.
Practically speaking, companies using Ethyl Imidazole-4-Carboxylate need robust supplier vetting. It’s not just about a COA (certificate of analysis). End users—especially those in pharma—demand in-depth QC records, process descriptions, and sometimes even evidence of cGMP compliance. This is where many commodity traders drop out, as they simply package and ship rather than produce on site or stand by their chain of custody.
Shortfalls often lead teams to either work with chemical brokers experienced in custom synthesis or invest in small-scale in-house production. The latter may involve significant upfront investment and lengthy regulatory navigation. I’ve seen institutions create internal incentives to support local, certified producers to reduce the risks tied to global uncertainty and shipping disruptions—a move that works, provided there’s a cooperative relationship and shared technical standards.
Transparency from supplier to end user can resolve many headaches. Live tracking of each batch’s origin, batch testing for residual solvents, and upfront dialogue about reordering timelines take priority over projecting cost per kilo. The future progress of this chemical’s market probably rests on collaborative partnerships, targeted technical documentation, and a willingness to engage in real conversations, not just transactions.
Purity matters in every step of chemical production, but it tends to get overlooked unless someone has experienced the fallout. In chemical labs, an unexpected trace impurity can send a synthesis in the wrong direction. Ethyl Imidazole-4-Carboxylate, often found in pharmaceutical research and specialty chemical processes, falls into that category: the purity isn’t just a number, it shapes outcomes. Typical suppliers specify a purity level of at least 98%. Some push that up to 99%, recognizing that research and drug development need fewer unknowns.
Anything less than this level and strange peaks start showing up in chromatograms. Instead of a simple reaction route, you’re troubleshooting side-products or conducting more purification. Even if a lab is only running pilot batches, those impurities can skew yields or cloud analytical results. Researchers look for ethyl imidazole-4-carboxylate that comes with a COA, showing each batch has passed HPLC or GC checks. In practice, that means checking for water content, ash, and related impurities, sometimes below 0.5% for each. These specs won’t just protect sensitive reactions—they help maintain consistent results project after project.
Some suppliers claim ultra-high purities, but what really counts is batch reliability. A packed schedule means nobody wants to purify a supposedly pure chemical for every experiment. Over the years, I’ve kept a list of trusted vendors based on how rarely their ethyl imidazole-4-carboxylate failed quality checks. It’s remarkable how a single out-of-spec batch can bring an entire workflow to a halt, especially when downstream chemistry depends on it. Most labs run a quick NMR or melting point just to make sure, regardless of certificates. The 98% figure doesn’t mean shortcuts: polyethylene-lined drums or narrow-necked bottles keep the product dry, since water uptake and degradation both matter.
Clear labeling and batch traceability can be overlooked, but not in places under regulatory strains. Production teams keep track of origin, lot number, and manufacturing date. This doesn’t just help track down sources if something goes off; it reassures regulatory bodies, too. It doesn’t matter if you’re working in a small-scale research lab or at a pilot plant—strong paperwork and reliable suppliers pay off in saved time and peace of mind.
Factories can struggle with consistency, mostly if equipment is switched over for different runs or solvents aren’t fully removed. Better training and robust SOPs—from storage temperature to drum cleaning—keep impurities in check. Finally, direct communication with suppliers works wonders. Asking pointed questions about analytical methods or impurity profiles has helped my teams filter out low-quality sources early, long before any headache hits the bench. Anyone sourcing ethyl imidazole-4-carboxylate for regulated or research uses learns quickly: purity is never just a number, and consistent quality keeps work moving forward.
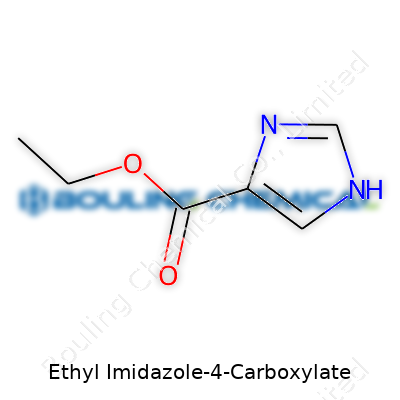

| Names | |
| Preferred IUPAC name | ethyl 1H-imidazole-4-carboxylate |
| Other names |
4-Imidazolecarboxylic acid ethyl ester Ethyl 1H-imidazole-4-carboxylate Ethyl imidazole-4-carboxylate Ethyl 4-imidazolecarboxylate |
| Pronunciation | /ˈɛθɪl ɪˌmɪdəˌzoʊl fɔːr kɑːrˈbɒk.sɪ.leɪt/ |
| Identifiers | |
| CAS Number | 87116-89-0 |
| Beilstein Reference | 88234 |
| ChEBI | CHEBI:85769 |
| ChEMBL | CHEMBL2007617 |
| ChemSpider | 22245822 |
| DrugBank | DB07925 |
| ECHA InfoCard | 03f7e2e2-175a-4187-a1cc-7c9e79e1250a |
| EC Number | 7659-86-1 |
| Gmelin Reference | 82194 |
| KEGG | C16235 |
| MeSH | D017175 |
| PubChem CID | 298217 |
| RTECS number | NK7175000 |
| UNII | M08J72FJ1B |
| UN number | UN2810 |
| Properties | |
| Chemical formula | C6H8N2O2 |
| Molar mass | 139.15 g/mol |
| Appearance | White to light yellow crystalline powder |
| Odor | Odorless |
| Density | 1.203 g/cm3 |
| Solubility in water | Slightly soluble |
| log P | 0.07 |
| Vapor pressure | 0.0116 mmHg at 25°C |
| Acidity (pKa) | 7.1 |
| Basicity (pKb) | 7.46 |
| Magnetic susceptibility (χ) | -59.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.516 |
| Dipole moment | 4.45 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 359.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -2069 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315: Causes skin irritation. H319: Causes serious eye irritation. H335: May cause respiratory irritation. |
| Precautionary statements | P261, P280, P305+P351+P338, P304+P340, P312 |
| NFPA 704 (fire diamond) | 1-2-0-NULL |
| Flash point | Flash point: 113.5 °C |
| Autoignition temperature | 310°C |
| Lethal dose or concentration | LD50 (oral, rat): >5000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat >2000 mg/kg |
| NIOSH | NIOSH: Not listed |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 10 mg/m³ |
| Related compounds | |
| Related compounds |
Imidazole Imidazole-4-carboxylic acid Methyl imidazole-4-carboxylate Ethyl imidazole-2-carboxylate 1-Methylimidazole-4-carboxylate |