The journey into the study of Ethyl 5-Oxo-L-Prolinate stretches back to the mid-twentieth century, when scientists keen on expanding the toolkit of organic chemistry started exploring derivatives of proline. Early researchers liked to tinker in their labs, working out novel synthesis pathways for amino acids and their esters, drawn by the lure of unlocking new functions for both medicine and industry. By the time chromatography caught on in research circles, this compound started showing up in a range of reaction products, prompting questions about its potential value in biochemistry and pharmacology. Labs kept up the search, refining preparation techniques, and eventually, a solid base of knowledge formed, laying out the structure and possible uses for this relatively niche ester.
Ethyl 5-Oxo-L-Prolinate stands out as an ester derivative of L-proline, one of the major natural amino acids. As someone with a chemistry background, I appreciate how it moves beyond the basic building block, offering synthetic chemists a platform for further transformation. It falls into a class of compounds valued for their reactivity as intermediates in organic synthesis. Chemists in the pharmaceutical sector often turn to this ester when designing drugs or testing new methods to tweak biological activity. Rather than being limited to the rarefied world of academic research, it’s popped up in specialty catalogs, getting used by startups and established firms alike to create analogs with improved properties for therapeutic use. This flexibility gives it an edge over simple amino acids.
From the physical side, Ethyl 5-Oxo-L-Prolinate appears as a clear to slightly amber liquid, sometimes showing up as a pale solid in colder environments. The molecular formula C7H11NO3 gives a manageable molecular weight that synthetic chemists find agreeable, especially for scaling up reactions. It shows moderate solubility in polar organic solvents such as ethanol and acetonitrile, and dissolves reasonably well in water thanks to its ester and lactam functionalities. Its melting point can hover near room temperature but shifts depending on purity and storage conditions; this can catch people off guard in labs without strict temperature controls. The compound comes with a characteristic odor, a subtle sign that someone’s working with delicate nitrogen compounds.
Quality assurance in chemical manufacturing hinges on clear labeling and technical standards. Suppliers usually report purity via HPLC, targeting 98% or greater to satisfy stringent research and commercial demands. Lab techs receive details about storage, often recommending refrigeration between 2-8°C to avoid any slow hydrolysis. Shipping labels highlight the ester’s flammability, and every bottle arrives with a safety data sheet. Some companies codify batches by lot number and date of manufacture, making life easier for researchers needing to track sources. Transparency on origins and purity means less second-guessing later in the workflow.
The classic preparation of Ethyl 5-Oxo-L-Prolinate begins with L-proline, a mainstay in many peptide synthesis labs. Chemists introduce the ethyl group through an esterification reaction, typically using ethanol in an acidic environment—think sulfuric acid or a more benign alternative like p-toluenesulfonic acid. To form the oxo group at the fifth position, oxidation takes place under controlled conditions, often relying on a precise oxidant to avoid overreaction or unwanted side products. Skilled hands in the lab monitor temperature closely, as overheating risks the integrity of the prolinate ring. Once the synthesis wraps, purification usually runs through silica gel chromatography, producing a clean compound, as judged by TLC and NMR. Waste from these reactions needs careful handling, something experienced crews manage to minimize ecological impact.
Once synthesized, Ethyl 5-Oxo-L-Prolinate becomes a springboard for a wide array of downstream reactions. The lactam functionality at its core encourages nucleophilic attacks, letting chemists build larger, more complex molecules from this starting point. In practice, I’ve seen the compound trusted in the synthesis of heterocycles, where its dual reactivity (domain of both the ester and the lactam group) unlocks multi-step routes to targets like substituted pyrrolidines and peptide-like fragments. Reduction steps turn the oxo group into an alcohol, supplying a different array of tools for further coupling reactions. With the right care, it’s possible to swap out the ethyl ester for bulkier alcohols, tuning the steric environment and exploring new reaction outcomes. Its role as an intermediate testifies to the creativity that chemists apply when chasing novel drugs or specialty materials.
The literature holds a few names for this compound, each hinting at its variety of uses. Ethyl 5-Oxo-L-Prolinate often shows up in catalogs as "ethyl 2-(ethoxycarbonyl)pyrrolidine-2-one-5-carboxylate." Some researchers call it "Ethyl pyroglutamate," a strand carried on from times when systematic naming felt less important than practical recognition. Commercial suppliers sometimes assign in-house codes to manage inventory, but in published research, the full IUPAC name keeps confusion at bay. For database searching, consistent synonym mapping plays a huge role.
Labs keep strict routines when handling Ethyl 5-Oxo-L-Prolinate, mostly due to its moderate flammability and risk of irritation from liquid splashes or vapors. Gloves, goggles, and fume hoods remain non-negotiable. In industrial settings, closed-system transfers help prevent accidental exposure or spills. The safety data sheet spells out serious risks—eye and respiratory irritation sit high on the list—but I’ve found seasoned chemists rarely experience trouble so long as best practices guide their work. Waste collection relies on solvent-safe containers, with local regulations dictating final disposal. Regulatory standards require documentation and training, keeping lab managers on their toes and reinforcing the mantra that skill, not just caution, guarantees smooth operations.
In my experience, Ethyl 5-Oxo-L-Prolinate earns its keep in the pharmaceutical research world. Medicinal chemists adopt it to tinker with proline-based drug structures, tweaking properties to hunt for oral activity or metabolic stability. Beyond medicine, material scientists use it to construct polymers with built-in flexibility or unique degradation behaviors. Biochemists exploring enzyme mimics rely on the ester as a substrate analog, probing catalytic efficiency in modified active sites. It doesn’t just fit into one niche—bioorganic, pharmaceutical, and polymer chemists each see something different in its framework. Sometimes, specialty manufacturers employ it as a stepping stone to agricultural chemicals or food additives, blurring lines between science and everyday life.
Research efforts keep expanding, aiming to unlock uncharted uses for Ethyl 5-Oxo-L-Prolinate. Recent publications sketch out its incorporation into peptidomimetic molecules, shining a light on possible routes for new drug candidates that challenge traditional bioavailability limits. Custom modifications—such as changing the length or shape of the ester group—open experimental vistas for exploring structure–activity relationships. Collaboration between academic groups and industry clusters brings in more hands and minds, leveraging both basic theory and commercial savvy. Funding bodies know that unique intermediates like this one can trigger real innovation and economic growth. Researchers keep an eye on reaction yields, seeking efficiency gains that lead to greener, cheaper, and faster processes. The pace of new publications and patents trace a healthy upward curve, suggesting lasting interest driven by practicality and vision.
Toxicity studies anchor the responsible use of Ethyl 5-Oxo-L-Prolinate. Lab tests in cell cultures and animal models collect data on acute and chronic effects. Early toxicology screens show limited systemic toxicity at low concentrations, yet repeated exposure or unprotected contact can result in irritation or allergic responses in sensitive individuals. Environmental persistence poses a less pronounced concern compared to halogenated compounds, but best-practice disposal keeps labs from slipping into regulatory headaches. Manufacturers rely on clear safety thresholds and recommend medical checks for those exposed over years of work. Ongoing research explores not just short-term impacts but possible metabolic byproducts, tuning risk assessments as new information lands. Ultimately, the safety profile supports confident use in controlled environments, though clinical-grade applications always warrant another round of testing prior to approval.
Looking ahead, Ethyl 5-Oxo-L-Prolinate sits in a sweet spot for future innovation. Advances in automation and green chemistry tweak old preparation methods, cutting costs and waste. Drug developers push harder into the landscape of unnatural amino acids and their derivatives to break through persistent problems in oral drug design. As diagnostics turn to more sensitive and specific assays, chemists find new incentives to invent modifications that improve binding or detection. Materials scientists eye its structure as a bridge to create “smart” polymers that react to stimuli—a field still taking shape but full of promise. The appetite for effective, sustainable syntheses will determine the pace of adoption, but current trends point to a broadening set of applications in pharmaceuticals, biotech, and specialty chemicals. Experience says that tools with flexibility, safety, and a proven track record often stick around and shift the way problems get solved.
Ethyl 5-Oxo-L-Prolinate might sound like something reserved for a chemistry textbook, but its role stretches well past the lab bench. What first caught my attention is its connection to the world of pharmaceutical research and drug design. Scientists use molecules like this as building blocks. These rare compounds sit right at the crossroads of biochemistry and innovation, where new medicines and treatments begin life.
Let’s start with the fact that Ethyl 5-Oxo-L-Prolinate is part of a family called proline derivatives. These molecules show up again and again during the design of synthetic drugs, especially those that target enzymes. Proline, at its core, influences the shape of proteins—which is crucial because a change in shape can flip a disease on or off. That sends ripples through drug discovery. Tiny tweaks using a molecule like Ethyl 5-Oxo-L-Prolinate might dramatically alter a new medicine’s effectiveness or safety.
This molecule doesn’t just matter to researchers in white coats. Picture the millions of people worldwide dealing with chronic pain or neurodegenerative disorders. When chemists make new versions of old medicines or search for something better, they often reach for proline derivatives as a core ingredient. I’ve read studies showcasing Ethyl 5-Oxo-L-Prolinate’s involvement in creating newer painkillers or memory boosters. This sort of progress brings hope, especially for families who have run into walls with standard drugs.
Though its fame starts in health research, the reach of Ethyl 5-Oxo-L-Prolinate can extend to agrochemicals and specialty materials. Farmers depend on better, safer pesticides. Modern materials scientists look for specialized building blocks to add new properties to plastics or coatings. All of these industries draw from a similar chemical toolkit, and this compound has proven itself useful for making things with pinpoint accuracy.
Handling chemicals isn’t routine for most people, but labs taking on Ethyl 5-Oxo-L-Prolinate have to keep a close eye on lab safety. Accidental misuse or poor disposal puts people at risk, and nobody wants harmful byproducts in local waterways. Chemical suppliers and universities put a premium on clear safety data and responsible sourcing. Behind every scientific advance, there’s a chain of people committed to safe, ethical practices. That’s sometimes lost in the headlines, but it deeply matters. Trained researchers benefit from timely guidance, and strict rules keep both workers and the community protected.
Full transparency in the research and sale of chemical agents remains top priority in today’s world. Information—clear, updated, and open—lets scientists collaborate across countries and industries. For people like me, who rely on trustworthy research to form opinions about drug safety and manufacturing, seeing open data is a good sign. I see value in continued investment in public health training and government oversight. The faster we swap knowledge in this field, the sooner everyone benefits.
Ethyl 5-oxo-L-prolinate may sound like something from a research paper, but it carries practical meaning in both academia and the pharmaceutical industry. Its molecular formula, C7H11NO3, isn’t just a jumble of numbers and letters. It sums up the count of each atom involved—seven carbons, eleven hydrogens, one nitrogen, and three oxygens. Every detail in that formula plays a direct role in how this compound acts, how it interacts, and where it fits into real-world applications.
Let’s roll back a bit. Ethyl 5-oxo-L-prolinate is an ester derivative of L-proline, itself an important amino acid. L-proline pops up all over biology—think proteins, enzymes, or even the creaky cartilage that cushions your knees. Adding an ethyl group and oxygen atom does more than just tweak a name; it changes everything about how this molecule behaves. That’s why formulas like C7H11NO3 aren’t some trivial factoid.
A simple switch—changing a carbon or oxygen—can spell the difference between a helpful pharmaceutical intermediate and a dead end in drug synthesis. Misreading or miswriting molecular formulas in the lab can lead to wasted time, botched experiments, or even safety hazards. I’ve seen it in working labs: a single number off the mark upends everything. So, remembering and understanding these formulas offers not just readiness for exams, but confidence in handling real compounds.
Down-to-earth experience tells me: chemistry, as much as it offers endless theoretical fun, likes precision. Academic researchers and pharma companies lean heavily on compounds like Ethyl 5-oxo-L-prolinate. A student tackling organic synthesis might use it to build new drug candidates. A chemical supplier needs to guarantee each batch is correct down to those atomic details. One missed count and the whole synthesis pipeline can falter.
This makes formula literacy practical. Consider regulatory review: authorities want a clear, reliable molecular identity attached to every lab report, every delivered gram, every patent claim. Getting the formula right helps avoid regulatory delays and batch recalls. The right numbers keep careers and businesses on track.
It’s not only chemists who benefit. Pharmaceutical quality inspectors, educators, and students all run into molecular formulas. I’ve watched educators hammer home the importance with hands-on models. Visualizing it—counting those carbons and hydrogens—turns rote learning into practical sense. In healthcare, patients gain from this precision too. Every time a generic drug hits the market, its active ingredient must share an exact formula and structure with its branded counterpart.
Chemists push science farther by settling on accurate, universally accepted formulas. Databases, journals, suppliers—everyone syncs up when the details match. It’s a small thing, yet it threads through innovation, safety, and global trust. No shortcut beats studying the details and learning the formula—C7H11NO3—for what it is and why it matters.
Labs, pharmaceutical plants, and research teams often take chemical purity as seriously as a chef checks the quality of their ingredients. Ethyl 5-Oxo-L-Prolinate is no different. Sourcing this compound opens the door to a conversation about purity grades, costs, and what the customer is really after. One might see grades labeled as “technical”, “analytical”, or “pharmaceutical”—each hinting at a specific range of impurities and suitability for use.
Pharmaceutical companies push for compounds with the lowest traces of contaminants. They want fewer unknowns because even a trace element in a batch can affect outcomes in preclinical testing. In my own experience helping friends in quality assurance, I’ve seen how rigorous their documentation becomes when purity creeps above 98%. Anything lower usually falls into the hands of chemical researchers. Some of these folks tweak molecules for further synthesis, or they use them as intermediates, so trace byproducts don’t always matter.
Not all chemistry is equal, and the stakes change depending on where and how a compound is used. For example, if a research group tests a molecule’s biological activity, the unexpected effects of an impurity might lead to wasted effort or wrong conclusions. A former colleague once ran a whole series of cell assays, only for the results to fall apart once the source of a contaminant was found—costing time and blowing the research budget.
On the other hand, a production chemist working at scale for, say, an industrial resin, can usually take a bit more leeway. The priorities shift from pinpoint accuracy to the price tag, since using ultra-pure grades can make a project so expensive that it stops making business sense.
Suppliers aiming for trust will print certificates of analysis (CoA) that list exact percentages. Reliable companies know that listing “99% pure” is only as good as the lab doing the measurement. Analytical methods like HPLC, NMR, or MS draw a bright line under the real numbers. I’ve noticed that researchers will often call up suppliers directly, seeking reassurance about what “grade” means in practical terms, since the buyer may be fishing for hidden details on isomeric purity or water content.
There’s a shared frustration across the industry with businesses that treat all chemical purchases as equal. I once watched a university lab lose a grant because a discrepancy between expected and delivered purity caused confusion in published results. Simple mistakes in chemical sourcing trip up entire projects. Cheaper grades might save a few dollars up front, but labs often end up re-running experiments or facing delays, which might mean a reporter or reviewer questions their findings.
It seems fair to push for more transparency from suppliers. Digital platforms that let customers scan certificates and batch data build more trust. Researchers should cross-check certificates, even if it slows them down, instead of blindly trusting a catalog listing. Regulators and standards bodies could step in to set minimum reporting requirements for suppliers dealing with sensitive research, public health, or medicine. Separating compounds by application, and spelling out what’s in the bottle, helps everyone in the long run.
Anyone who has spent time working with specialty chemicals like Ethyl 5-Oxo-L-Prolinate knows that proper storage isn’t just a housekeeping matter; it’s a health and safety commitment. I’ve seen what happens when someone leaves a sensitive compound on a bench under fluorescent lights for a few days—what starts as a useful substance sometimes ends up useless or worse, potentially dangerous.
Ethyl 5-Oxo-L-Prolinate has the same quirks many organic esters have. It prefers a cool, dry space much more than a warm, humid shelf in a general supply closet. Too much moisture from the air, and you’ll start to see degradation before a single experiment kicks off. That isn’t paranoia; it’s chemistry. Hydrolysis can quietly convert the ester, releasing byproducts that might throw off results or, at scale, even pose real hazards.
A storeroom that holds steady at 2–8°C (basically a standard refrigerator or a temperature-controlled cabinet) keeps ethyl 5-oxo-L-prolinate stable. Most glass ampules or plastic containers with tightly sealed lids work well, as long as you avoid cap types that warp or loosen with time. Even the best chemical loses quality if moisture or oxygen sneaks in.
It pays to keep the compound away from strong light. I learned early that even some fluorescent fixtures can influence the stability of certain reagents. Light can kick off side reactions in some molecules, and watching an entire batch degrade into a gummy mess isn’t something you forget. Amber glass bottles help a lot, but light exposure still counts through the day—closed cabinets or cardboard boxes provide the extra protection that pays off long-term.
Crowding the shelves with incompatible reagents never ends well. Acids, strong bases, or oxidizers stored next to Ethyl 5-Oxo-L-Prolinate will eventually cause trouble. I recall a particularly unfortunate incident in a shared university lab: an improperly capped bottle of concentrated hydrochloric acid nearby led to pitted aluminum foil and a suspicious odor by the weekend. Better to give every chemical its zone and double-check bottle labels every week.
A simple label with the date received and first opened goes a long way. Chemicals age, and even with all the right conditions, nothing lasts forever. I mark the storage date and always jot down my initials. If the label’s sticky residue builds grime, transfer the info to a fresh label. This habit spared me from using a reagent that silently expired before its time.
It’s tempting to order extra for the sake of savings, but oversized quantities almost always outstay their welcome. Smaller containers mean fewer headaches about degradation and safer disposal later on. Outdated or decomposed chemicals shouldn’t just hit the trash. Most cities and universities offer hazardous waste streams, and it’s critical to use them—one spill can cost more in lost time and clean-up than the reagent ever did.
In my years working in both academic and industry labs, the simplest systems worked best: low temperatures, low humidity, light-protected, separated by hazard class, and always labeled. These steps keep Ethyl 5-Oxo-L-Prolinate ready for honest, reproducible chemistry whenever you need it.
Staring at a lab bottle marked Ethyl 5-Oxo-L-Prolinate brings a mix of curiosity and caution. It’s a synthetic molecule, used in specialty synthesis, and its structure hints at reactivity. Despite its interesting chemistry, skipping over safety can spell trouble.
I’ve worked with dozens of esters and amides, and one lesson always stands out: chemicals demand respect. The clear, sweet-smelling liquids mask hazards. Even a small splash causes skin irritation. Years ago, I underestimated a similar compound; the sting that followed became a permanent reminder.
Ethyl 5-Oxo-L-Prolinate causes skin and eye irritation. Inhaling dust or vapors gives headaches or breathing trouble. Swallowing it leads to nausea or more serious symptoms. Glove use isn’t just a checkbox. Nitrile gloves provide a barrier, stopping the substance from soaking into the skin. Lab coats stop splashes from spreading and keep your own clothes uncontaminated.
Eye protection matters, too. Standard goggles become second nature after seeing a colleague narrowly avoid permanent eye damage during a routine transfer. Spilled drops can jump where you don’t expect.
Storing the bottle on a high shelf or next to acids risks trouble. A labeled, corrosion-proof cabinet stops mixing accidents, and keeping the cap tightly sealed slows off-gassing and evaporation. Good ventilation makes a huge difference. Fume hoods might sound over the top for powders or liquids like this, but the peace of mind is worth those extra steps.
Accidents still happen. Paper towels never cut it with synthetic esters; they spread the mess and let solvent vapors linger. Absorbent pads, followed by thorough rinsing with water, control the aftermath. Waste always goes into a labeled, chemical-specific bin—I learned the hard way that chemical waste, left in a generic trash can, creates unexpected hazards.
Making safety standard in every group session means talking about real risks and showing the right techniques. Printed safety data sheets belong in arm’s reach. No lab mate should wonder what to do during a spill or exposure. Routinely reviewing safety protocols prevents someone from cutting corners under pressure.
Simple routines make labs safer. Gloves by the door, goggles hanging within sight, spill kits on hand—all these steps take little effort but prevent disasters. Regularly checking airflow, reviewing container labels, and reminding everyone of the common-sense rules make a culture of safety stick.
Chemistry moves fast, and new compounds appear often. Respect for each unfamiliar bottle matters just as much as expertise in the reaction itself. The best labs share more than data—they build habits that keep everyone safe. Ethyl 5-Oxo-L-Prolinate might never become famous outside the synthetic chemistry world, but treating it with care means the next discovery isn’t overshadowed by an avoidable accident.
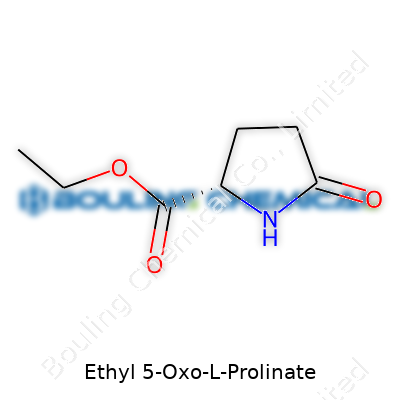
| Names | |
| Preferred IUPAC name | ethyl (2S)-5-oxopyrrolidine-2-carboxylate |
| Other names |
Ethyl (S)-5-oxo-2-pyrrolidinecarboxylate Ethyl (S)-5-oxopyrrolidine-2-carboxylate Ethyl 5-oxo-L-pyrrolidine-2-carboxylate |
| Pronunciation | /ˈɛθɪl faɪv ˈɒk.səʊ ɛl prəʊˈlɪ.neɪt/ |
| Identifiers | |
| CAS Number | [867-91-2] |
| 3D model (JSmol) | `3D model (JSmol) string` of **Ethyl 5-Oxo-L-Prolinate**: ``` C[C@@H]1CC(=O)NC(=O)O1CC ``` *(Note: This is the SMILES string representation, commonly used for JSmol input of 3D models.)* |
| Beilstein Reference | 109184 |
| ChEBI | CHEBI:68452 |
| ChEMBL | CHEMBL3702406 |
| ChemSpider | 141547 |
| DrugBank | DB08339 |
| ECHA InfoCard | 07f2084b-41c8-462c-9707-54a5f716065b |
| EC Number | Ethyl 5-Oxo-L-Prolinate" does not have an assigned EC Number. |
| Gmelin Reference | Gmelin Reference: **83748** |
| KEGG | C03189 |
| MeSH | D017855 |
| PubChem CID | 11526527 |
| RTECS number | KY8775000 |
| UNII | L71518TF6N |
| UN number | UN3272 |
| CompTox Dashboard (EPA) | DTXSID4041269 |
| Properties | |
| Chemical formula | C7H11NO3 |
| Molar mass | 157.17 g/mol |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | 1.169 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -1.3 |
| Vapor pressure | 0.06 mmHg at 25°C |
| Acidity (pKa) | 7.43 |
| Basicity (pKb) | 8.75 |
| Magnetic susceptibility (χ) | -45.1 · 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.481 |
| Viscosity | 52 cP |
| Dipole moment | 3.27 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 365.9 J⋅mol⁻¹⋅K⁻¹ |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | `GHS07` |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | Precautionary statements: P210, P233, P240, P241, P280, P303+P361+P353, P370+P378 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | Flash point: 143.1 °C |
| Autoignition temperature | Autoignition temperature: 385 °C |
| Lethal dose or concentration | Lethal dose or concentration not found. |
| LD50 (median dose) | LD50 (median dose): Oral, mouse: 3240 mg/kg |
| NIOSH | Not established |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 mg/m³ |
| Related compounds | |
| Related compounds |
L-Proline 5-Oxo-L-proline Ethyl L-prolinate Proline methyl ester N-Acetyl-L-proline |