Ethyl 4-piperidinecarboxylate stems from the broad family of piperidine derivatives that have shaped organic chemistry since the early 1900s. Early synthetic chemists quickly realized that five- and six-membered nitrogen rings offered a springboard for making pharmaceuticals, agrochemicals, and advanced materials. Over the decades, labs chased down better synthetic routes to piperidine derivatives, opening doors for specialty chemicals like Ethyl 4-piperidinecarboxylate. Researchers, inspired by milestones like the discovery of piperidine’s natural occurrence in black pepper, dug into carboxylation and esterification methods. From these seeds, modern preparation techniques for Ethyl 4-piperidinecarboxylate took root, reflecting a story of slow, methodical innovation rather than flashes of genius.
A clear, colorless to pale yellow liquid, Ethyl 4-piperidinecarboxylate appeals to process chemists looking for a versatile building block. Its compact heterocyclic structure—piperidine ring bearing an ethyl ester group—is no accident, combining the stability of aliphatic nitrogens with the functionality that modern chemistry demands. This material doesn’t just sit on the shelf; its reactivity and compatibility with varied solvents have made it a go-to in labs that stock materials judged by more than simple availability or price.
Ethyl 4-piperidinecarboxylate features typical alkyl ester attributes and a six-membered nitrogen ring. It registers a molecular weight of about 171.24 g/mol and sits at a boiling point above 200°C, making it useful for high-temp synthesis. Chemists appreciate its modest water solubility, which strikes a balance for both organic and aqueous systems. The density hovers near 1.02 g/cm³ at room temperature, packing enough molecular heft to handle yet not so much as to suffer viscosity issues in production lines. Its chemical stability, even in air, cuts down on waste and makes accidental degradation a rare concern.
Bottles of Ethyl 4-piperidinecarboxylate come with essential details, not marketing jargon. Standard labels document purity—typically above 98%—trace metal analysis, batch number, and the expiration date. Determining authenticity matters; industrial buyers check for CAS number 4549-70-0 and verify compliance with global shipping regulations under UN identification. Data sheets usually spell out storage rules, encouraging cool, dry, well-ventilated spots away from incompatible oxidizers or acids. Reliable manufacturers also provide safety documentation in line with GHS, including all pictograms and hazard statements.
Laboratories prepare Ethyl 4-piperidinecarboxylate through classic esterification. Starting with 4-piperidinecarboxylic acid, chemists add excess ethanol and a catalytic amount of sulfuric acid or another dehydrating agent. Temperature control stays key: too high and you get byproducts; too low and the yield suffers. After reaction completion, neutralization and extraction steps follow, then distillation or column purification. This route gives high-yield, consistent product—after years of lab tweaks aimed at keeping costs down and purity up. In pilot plants, continuous reactors help scale this process safely, further reducing run-to-run variability. Improvements in greener synthesis—drawing on renewable solvents or recyclable catalysts—reflect growing pressure from both regulators and bottom lines.
The ethyl ester group on Ethyl 4-piperidinecarboxylate invites all sorts of transformations. Hydrolysis splits the ester back to its acid under acidic or basic conditions, giving access to functionalized intermediates. Reduction with hydrides brings alcohol derivatives within reach. Acylation and alkylation prove straightforward with its accessible nitrogen, and skilled chemists use this compound to spin out libraries of derivatives for pharmaceutical screening. In multi-step syntheses, its ring nitrogen can anchor protecting groups or act as a nucleophile for cyclization—opening doors to complex, pharmacologically active scaffolds that remain out of reach from simpler precursors.
In the trade and research world, the same compound picks up several aliases. Ethyl 4-piperidinecarboxylate goes by N-carboethoxy piperidine, 1-ethoxycarbonyl-4-piperidine, or simply EPC. Specialty suppliers may reference its registry number or use commercial names tied to their own branding. Chemists often abbreviate, and catalogues sometimes list the product as 4-piperidinecarboxylic acid ethyl ester. Knowing these names helps buyers avoid mix-ups, especially in projects juggling dozens of nitrogen heterocycles at once.
Anyone working with Ethyl 4-piperidinecarboxylate faces standard organic chemical hazards—especially skin and eye irritation, and respiratory risks at higher vapor exposures. Plants and labs lean on robust ventilation, nitrile gloves, and eye shields instead of hoping for luck. Safety Data Sheets demand prompt washing after handling, and spill procedures focus on dilution and absorption rather than just containment. Waste protocols call for licensed disposal teams, in line with regional environmental law, to avoid unauthorized dumping. In my experience, seasoned chemists treat every batch with respect, not just the ones flagged as “highly toxic” or “flammable,” because small exposures add up.
Ethyl 4-piperidinecarboxylate ends up in all sorts of chemical sectors. Medicinal chemists regard it as a scaffold for experimental drugs targeting neurological and psychiatric disorders. Agrochemical researchers have leveraged its ring system to design new pesticides and growth regulators, taking advantage of its metabolic stability. Polymer labs use it as a modifier for engineering plastics, enhancing flexibility without undermining strength. In specialized R&D, the compound often supports asymmetric synthesis projects, where even small efficiency wins can lead to patentable new routes. Its status isn’t hype; it’s earned by the way it can transform into more valuable and complex molecules, helping teams bridge the gap between whiteboard sketches and viable products.
Current research looks beyond just making more Ethyl 4-piperidinecarboxylate with slightly better yields. Teams dig into process intensification, squeezing out every percent of atom economy. Data-driven approaches let chemists map out the most promising reaction partners, cutting down trial-and-error cycles. There’s growth in finding new ligands and catalysts that unlock the full potential of its core ring. Researchers from pharmaceuticals to specialty materials appreciate the way this compound ties together diverse organic reactions, reducing the need for exotic starting materials and harsh conditions. Patents continue to cite new uses for its derivatives, especially as more researchers apply machine learning to unlock structure–activity insight.
Toxicologists have mapped out Ethyl 4-piperidinecarboxylate’s acute and chronic exposure impacts on model organisms. Initial findings show low acute oral toxicity in rodents at practical doses, but its metabolism can yield less-friendly byproducts, especially under repeated exposure or in combination with certain solvents. Lab rats exposed for months at a time showed signs of liver impact, pointing to the importance of thorough risk assessments before greenlighting it for drug or agrochemical pipelines. Regulatory agencies caution against spills into the water cycle; even low-level emissions need careful management to safeguard aquatic life and downstream users. These findings push labs to improve process containment and to substitute in greener alternatives whenever possible.
Ethyl 4-piperidinecarboxylate faces a landscape shaped by stricter chemical regulations, shifting consumer expectations, and the search for greener chemistry. Its adaptable structure keeps it in demand for those designing the next wave of faster-acting antidepressants, smarter pesticides, or lighter polymers. At the same time, innovation in catalyst design and continuous-flow synthesis will likely further decrease the environmental footprint of its production. Technology transfer from research to large-scale industrial settings now takes center stage, pushing firms to invest in smarter monitoring tools and process automation. Looking ahead, labs that master not just the chemistry, but also the transparency and traceability of their Ethyl 4-piperidinecarboxylate, will stay ahead in an industry that rewards both technical mastery and public trust.
Ethyl 4-piperidinecarboxylate never turns up in dinner-table chat, and I doubt you'll see it trending online. Yet, in chemistry labs, this compound is pretty familiar. The main reason? Its structure, built on a piperidine ring, makes it a handy starting point for making other chemicals. Think about the pharmaceutical industry, which depends on molecular scaffolds like this to build complex drugs. This compound supports development of treatments for stubborn health problems. It helps create molecules that go into drugs for pain relief, mental health disorders, and neurological conditions.
I remember watching a synthesis come together in a research setting. All those colorless liquids and white powders seemed dull until you realized the outcome might be a new way to fight pain or control seizures. Ethyl 4-piperidinecarboxylate brings in a mix of reactivity and practicality. Chemists rely on it to introduce the piperidine core—a backbone in medications such as certain opioids or antidepressants—into target molecules. The chemical’s simple structure allows researchers to "decorate" it with functional groups, shaping molecules useful for treating patients.
There’s another side to its story. In research, this compound steps in during chemical testing and analysis. Scientists use it as a reference or precursor for testing new reactions. For anyone who’s spent hours purifying compounds and checking their purity, this chemical helps speed things up. The predictability of its reactions turns it into a practical lab partner for anyone working with piperidine derivatives. Without it, many studies would stall at early stages, since synthesizing the piperidine ring from scratch can take extra time and resources.
Chemicals like this aren’t without issues. There’s always a dark side when it comes to research chemicals—someone somewhere thinks of ways to misuse them. Its structure, related to substances used in drug production, means regulators and suppliers stay watchful. The proper use of any chemical, especially those with medicinal value, comes down to responsible handling and oversight. Licensing, monitoring, and educating users in research settings protect both public health and scientific progress.
I’ve seen the best results in research when collaboration happens: chemists, pharmacists, doctors, and regulators all work together. Open communication keeps everyone alert to both opportunities and risks. Making sure supplies move only to verified labs reduces the risk of diversion. Education of staff in chemical safety prevents accidents and improper use. Teams benefit from checklists, inventory tracking, and clear rules for storage. With smart systems, labs get what they need for research, while communities remain protected.
Ethyl 4-piperidinecarboxylate plays a quiet but essential role in modern science. It powers discoveries in medicine and stands at the start of important new molecules. Used responsibly and stored securely, it helps move health care forward. Anyone working with such chemicals needs solid knowledge, care, and respect for the tools that drive scientific progress.
Ethyl 4-piperidinecarboxylate belongs to a family of organic compounds that mix basic chemistry with practical applications. Its formula isn't hard to remember: C8H15NO2. This means every molecule starts with eight carbon atoms, gets a backbone of fifteen hydrogens, nests a single nitrogen, and anchors in two oxygen atoms. Folks working in labs or even classrooms meet this name in textbooks or during hands-on synthesis work. Its structure packs more details than the formula alone shows.
This compound features a piperidine ring—a simple, six-membered cycle with five carbons and a nitrogen. One of the carbons on this ring, known as the four-position, carries a carboxylate group. By attaching an ethyl chain through an ester bond, the whole molecule takes on both flexibility and a big role in reactions. The ethyl piece often helps tune solubility and reactivity, making the compound handy in both academic research and the pharmaceutical industry.
The arrangement looks straightforward if you’ve seen cyclic amines before. The ring isn’t aromatic, so all atoms sit fairly evenly spaced. At the four-spot on the ring, a carboxylic acid group accepts an ethanol chain—this forms the “ethyl ester” side of the name. This bond lightens up processing, making it easier to convert the compound later or remix it into new molecules.
Anyone interested in organic chemistry or drug design will run into ethyl 4-piperidinecarboxylate sooner or later. The piperidine structure often pops up in painkillers, antipsychotics, and muscle relaxants. Chemists use the ester variant to help with purification or as a starting point in multi-step syntheses. Its basic ring system acts as a building block—plug in side chains and those base properties can shift into wholly different directions.
In some labs, researchers rely on this molecule to introduce piperidine features into more complex drugs. The ester format offers plenty of space for reaction teams to tweak, so it stays popular in custom synthesis. Pharmaceutically, manipulation of this compound helps build libraries of candidate drugs with the right shape and charge to interact with living tissue. Changing the ethyl group or swapping positions on the ring brings new activity or changes metabolism patterns. That’s why it stays on the shelves of universities, CROs, and even major drugmakers.
Sourcing or making ethyl 4-piperidinecarboxylate isn’t without speed bumps. Handling nitrogen heterocycles takes attention—avoid strong acids or oxidizers without a second thought. Also, the ester group sometimes breaks down in water or strong bases, so you need good storage and handling protocols. I learned early in grad school that even a quick slip or using old solvent means having to start from scratch. If scale becomes an issue, making this compound in bulk can lead to waste or byproduct headaches unless processes follow green chemistry principles.
Researchers keep chasing ways to tweak this molecule. People experiment with swapping the ethyl group or modifying the ring’s positions. These variations may lead to new medical treatments, improved manufacturing protocols, or even safer pesticides. It’s clear from the literature and my talks with colleagues that attention to responsible sourcing, purity testing, and waste management will shape the future of this compound’s use. Safety and stewardship go hand-in-hand these days, especially when these molecules show up in both legitimate labs and, regrettably, sometimes in the wrong hands. Continued transparency and clear labeling help everyone use it right, ensuring advances outweigh risks.
Ethyl 4-piperidinecarboxylate isn’t a name most folks run across in daily life. Researchers and professionals in chemical labs know it through various synthesis pathways and paperwork, often as an intermediate for making other molecules. I remember seeing it on a printed reagent list during my university days, another complex name among dozens. These aren’t the products sold at the local drugstore, and few outside labs encounter them directly.
Safety always becomes a focus every time someone handles a new reagent. I’ve stood shoulder-to-shoulder with colleagues sharing a respect, even for substances that look benign. That respect often comes from reading safety data sheets long before unscrewing a cap. The MSDS lists ethyl 4-piperidinecarboxylate as an irritant: exposure to eyes and skin can cause problems, and no one should breathe in the vapors or dust.
Lab safety trainers tend to hammer in the message — many organic compounds may look harmless, but you don’t want to eat, inhale, or rub any chemical into your skin if you can help it. Guidelines say to use gloves, goggles, and keep it in a fume hood. Even if something “only” burns, it’s best not to find out how severe that burn could get.
Lots of chemicals, especially intermediates, don’t get the same level of public scrutiny or detailed toxicity studies as finished drugs. Direct, conclusive data on ethyl 4-piperidinecarboxylate toxicity in humans won’t be easy to find. The risk profile often depends on structure and similar compounds, which sometimes share traits like central nervous system effects or general irritancy. For this one, researchers tend to group it with other piperidine derivatives that can be toxic at high doses, but practical risk depends a lot on exposure level.
Accidental exposure cases seem rare or go unreported, but that doesn’t mean safety doesn’t matter. In my own work, simple caution with gloves, containers, and careful washing goes a long way. Chemical manufacturers reinforce this message, and people who cut corners usually end up with stories about hives, rashes, or hospital trips.
Best practices come from a mix of regulation, experience, and common sense. Labs store this kind of compound in well-marked containers, away from high-traffic zones. Ventilation matters—nobody wants accidental inhalation to sneak up in a crowded workspace. Cleaning up spills with the right absorbents saves a lot of skin, and quick access to eyewash stations or showers addresses mistakes as they happen.
Disposal presents another challenge. Toxic or potentially carcinogenic compounds can’t go down the drain. Licensed disposal companies handle chemical waste. It costs more, and companies invest in this because the environmental impact of improper dumping isn’t just about fines or bad PR. I’ve shared responsibilities for logging waste in online databases, and regulations carry real teeth.
Chemistry can sound daunting, but most risks feel manageable with solid habits. Every time researchers push to innovate—making new pharmaceuticals or materials—they rely on safe handling practices shaped by decades of incidents and improvements. Taking shortcuts rarely pays off. Honest conversations about the risks serve both the people handling the chemicals and the wider environment. Everyone deserves to go home safe after a day in the lab.
Anyone who spends time in a lab or manages a chemical inventory knows that some materials demand a lot more respect than others. Ethyl 4-Piperidinecarboxylate has joined that list for me, not just for its utility, but for the risks it brings. It’s easy to forget safety rules until you see what happens when somebody ignores them. Caution grows out of experience—especially in spaces where strong odors, irritations, or worse can happen in a blink.
Leaving chemicals in just any cabinet guarantees trouble. Humidity, heat, light—each factor can chip away at purity or create dangerous byproducts. If flammable solvents sit too close to heat or sparks, disasters start small but escalate fast. I’ve seen incidents where mislabeled bottles and casual stacking led to ruined experiments at best, and hazardous leaks at worst.
Ethyl 4-Piperidinecarboxylate is a clear liquid, but that doesn’t make it harmless. Its structure makes it both useful and potentially irritating if touched or inhaled. Without dry, cool storage, chemicals break down or build pressure. Some labels even point out instability above room temperature. Failing to read up on stability data from verified sources risks both personal safety and lab results.
Let’s keep it straightforward. Chemicals like this belong in tightly sealed containers, away from acids, bases, strong oxidizers, and anything that reacts with esters or amines. Whether you use glass or specific plastics, check compatibility. Avoid makeshift containers and faded labeling. Professional storage means clear hazard signs and visible expiration dates.
A temperature-controlled storage cabinet works best, especially one rated for flammable liquids. Think about the last time a fridge’s thermostat failed. Without regular checks, even a small leak can go unnoticed until it causes corrosion, odd smells, or crystalline deposits. Regular inspections catch problems early.
Lighting plays a bigger role than most expect. Some compounds break down when exposed to light for a long period, forming unknown mixtures. Doors on storage units ought to block out sunlight and stray UV from fluorescent lamps. Simple, well-fitted shelves prevent bottles from knocking together or tipping over.
With any chemical, a pair of nitrile gloves, safety goggles, and a lab coat form the bare minimum for personal protection. Even quick transfers from one vessel to another can cause splashes. Use fume hoods, not just open windows. I saw a careless pour cause coughing fits across a whole room—one whiff is enough to keep you careful for months.
Use spill trays and absorbents nearby. Install eye wash stations and showers in spaces with regular handling. Store emergency contact numbers, material safety sheets, and neutralizing agents where anyone can reach them. Do regular drills so no one panics when an accident happens.
Training can’t just run on autopilot. Review what to do with spills, inhalation, or skin contact at least twice a year. Log all chemical movements, and track inventory in detail, including disposal dates. Seek advice from chemical safety specialists or local agencies. If a product seems old, cloudy, or smells odd, treat it with suspicion, not routine.
Safe storage and handling go way beyond compliance. They draw a line between safe, productive work and emergencies that pull in fire departments, health clinics, and public scrutiny. Policies built on real experience and careful monitoring have saved more labs than luck ever will.
Ethyl 4-piperidinecarboxylate shows up on a lot of chemical suppliers’ lists, but most folks outside the lab never hear its name. I remember glancing over bottles labeled with strange names during a stint in an academic lab, never really realizing how these small molecules build out the scaffolding for all sorts of new creations. The reason many researchers work with Ethyl 4-piperidinecarboxylate starts with its piperidine ring: this ring appears in a countless number of biologically active molecules. Inside research labs, if a scientist is making a new drug candidate or trying to unlock a novel chemical pathway, it’s a fair bet a piperidine derivative will play a role somewhere.
Medicinal chemistry teams often use Ethyl 4-piperidinecarboxylate as a starting material, especially in the search for new central nervous system agents. Many well-known pharmaceuticals, from antipsychotics to antihistamines, include piperidine structures. Compared to building these rings from scratch, grabbing a ready-made version with convenient handles like an ethyl ester saves valuable days or even weeks. Dozens of published studies rely on Ethyl 4-piperidinecarboxylate as a base for synthesizing molecules that interact with neurotransmitter systems. Building on a scaffold that already matches the body’s needs gives research teams a head start in the race to solve hard biological problems.
Beyond drug research, this compound pops up in the manufacture of so-called fine chemicals. That includes flavors, fragrances, and specialty materials. Chemists look for building blocks that react predictably and can be modified on a small scale. Since piperidine rings help stabilize certain molecules and fit neatly into increasingly complex products, Ethyl 4-piperidinecarboxylate earns its keep by showing versatility. I saw colleagues use this very ester to prepare amides and acids; its balanced reactivity lets lab teams swap out parts of the molecule without too many unexpected side reactions. This flexibility matters since every extra purification step means more time and cost.
Not every research project using this chemical ends up as a new pill. Organic chemists need reliable building blocks to tinker with novel reactions or to test out catalysts. Some academic teams have explored Ethyl 4-piperidinecarboxylate’s reactivity to better understand hydrogenation processes or C–N bond formation. These findings sometimes filter into broader industrial innovation, even if the public never hears about them. As the foundation for library synthesis, the compound helps researchers build collections of small molecules, then screen them against everything from enzymes to crop pests.
Scaling up from milligrams in a lab flask to kilograms in a pilot plant can uncover hidden headaches. Odor, volatility, and cost start to matter much more once chemists head toward industrial scale. Good lab practice calls for goggles, gloves, and proper ventilation, because volatile organics like ethyl esters may cause irritation or pose health risks if mishandled. Safety data hangs prominently in every lab I’ve worked in—experienced chemists treat each ester with a mix of respect and routine.
The future leans towards greener processes, less chemical waste, and safer alternatives. Some research leaders suggest redesigning reactions to use less hazardous solvents, or tweaking structure to give the same synthetic routes with less environmental impact. There’s no single answer, but teams share new methods at conferences and in journals, inching toward smarter, cleaner chemical manufacturing. Reducing exposure to hazardous compounds remains a core goal, so safer substitutes or improved containment win every time someone can make them work.
Ethyl 4-piperidinecarboxylate rarely makes headlines, but its steady value in research and industry keeps doors open for new medicines, better materials, and improved lab techniques. It might not be glamorous—just ask the person who’s spent hours scrubbing glassware after a sticky ester prep—but its influence echoes across discovery and innovation.
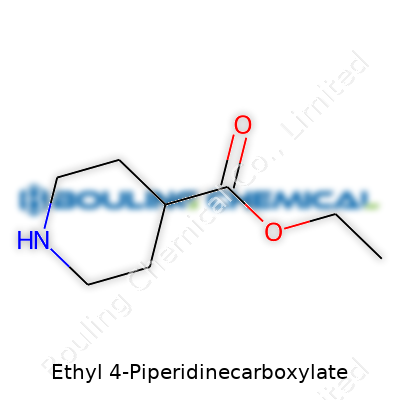
| Names | |
| Preferred IUPAC name | Ethyl piperidine-4-carboxylate |
| Other names |
Ethyl piperidine-4-carboxylate Piperidine-4-carboxylic acid ethyl ester Ethyl 4-carboxypiperidine 4-Piperidinecarboxylic acid ethyl ester |
| Pronunciation | /ˈɛθɪl ˈpɪpəˌriːdiːn kɑːrˈbɒksɪˌleɪt/ |
| Identifiers | |
| CAS Number | 4245-41-4 |
| 3D model (JSmol) | `3Dmol('CCOC(=O)C1CCNCC1')` |
| Beilstein Reference | 136607 |
| ChEBI | CHEBI:131303 |
| ChEMBL | CHEMBL16200 |
| ChemSpider | 79168 |
| DrugBank | DB03722 |
| ECHA InfoCard | 03b2a160-15c1-4680-8e78-5d31cdfb7b9b |
| EC Number | Ethyl 4-Piperidinecarboxylate" does not have a designated EC Number. |
| Gmelin Reference | 82117 |
| KEGG | C14399 |
| MeSH | D010903 |
| PubChem CID | 69124 |
| RTECS number | UY2275000 |
| UNII | Z7B83Y035F |
| UN number | UN3271 |
| Properties | |
| Chemical formula | C8H15NO2 |
| Molar mass | 157.22 g/mol |
| Appearance | Colorless to light yellow liquid |
| Odor | Characteristic |
| Density | 1.056 g/cm3 |
| Solubility in water | Slightly soluble |
| log P | 1.0 |
| Vapor pressure | 0.3 mmHg (25°C) |
| Acidity (pKa) | 10.8 |
| Basicity (pKb) | 2.97 |
| Magnetic susceptibility (χ) | -55.6e-6 cm³/mol |
| Refractive index (nD) | 1.456 |
| Viscosity | 60 mPa·s (20 °C) |
| Dipole moment | 4.07 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 348.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -393.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3661 kJ/mol |
| Pharmacology | |
| ATC code | N06AX19 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes serious eye irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P272, P273, P280, P302+P352, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362+P364, P501 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | 63°C |
| Lethal dose or concentration | LD50 (oral, rat): 500 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 430 mg/kg |
| NIOSH | UE2275000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | No REL established |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
Piperidine 4-Piperidinecarboxylic acid Ethyl piperidine-4-carboxylate hydrochloride Methyl 4-piperidinecarboxylate 1-Boc-4-piperidinecarboxylic acid 4-Cyanopiperidine 4-Hydroxypiperidine |