Chemists started turning to piperidine structures in the late 19th century, tracing back to a period when medicinal chemistry began shifting focus from plant-derived compounds to synthetic molecules. French chemist L. Knorr, who dabbled in heterocyclic chemistry, explored piperidine rings early on, pivoting them into a cornerstone for pharmaceuticals. Ethyl 4-Hydroxypiperidine-1-Carboxylate gained traction as researchers realized the impact of functionalizing piperidine rings. In the 1980s, with spectrometry tools and better reaction controls, the path opened wider for synthesizing variations like this one, especially for medicinal use and as a building block in fine chemical manufacture.
Ethyl 4-Hydroxypiperidine-1-Carboxylate catches the interest of laboratory scientists and manufacturing engineers alike. It looks innocuous at first—usually a white or off-white crystalline powder—but it harbors a reactive mix of features. Market demand for this compound grows primarily from pharmaceuticals, agroscience, and, increasingly, high-end material science. Being a versatile intermediate, its functional groups enable synthesis of diverse molecules, including certain specialty drugs, crop protection compounds, and additives in polymer production.
This compound doesn’t give off much smell. Powder at room temperature, it melts at around 74–78°C and dissolves in common organic solvents, such as ethanol, chloroform, and a bit less in water. Molecular weight clocks in near 188 grams per mole. Its structure, featuring an ethyl ester and a hydroxy group at the fourth carbon of the piperidine ring, combines stability with useful reactivity: the hydroxy group opens doors for further reactions, whereas the ethyl ester withstands moderate reaction conditions.
Chemical producers selling this compound keep purity high, often over 98% determined by HPLC. Typical containers are labeled with batch number, production date, storage instructions—usually keep cool and dry—and hazard statements in line with GHS regulations. Shipment follows guidelines for organic chemicals, with Material Safety Data Sheets (MSDS) required for large quantities. Regulatory compliance ensures traceability, crucial in pharmaceutical manufacturing.
Synthesis relies on several steps: usually starting from piperidine, which undergoes hydroxy functionalization at the fourth position—by oxidation or direct substitution—before carboxylation at the nitrogen. Often, ethyl chloroformate serves as a carboxylating agent. What matters in scale-up is yield, purity, and minimization of byproducts. Reaction conditions, especially solvent choice and temperature, influence both efficiency and safety. Analytical QC checks run throughout, using NMR and LC-MS to confirm identity and spot impurities.
Few molecules offer the same options for modifications. The 4-hydroxy group allows alkylation, acylation, or even conversion to halides for further transformations. The ester group, in the presence of acid or base, gets hydrolyzed to give the free acid—another useful intermediate. Large-scale chemists take advantage of this reactivity to introduce various substituents, tailoring downstream compounds to suit specific drug profiles, crop science agents, or even new polymers.
Chemical suppliers and regulatory bodies recognize it under several names. Some call it Ethyl 1-Carboethoxypiperidin-4-ol. Others use 4-Hydroxy-1-piperidinecarboxylic acid ethyl ester, sometimes short-handed as EHPCE or E4HPCE in specialty catalogs. Keeping track of these synonyms matters, especially in global procurement where regulatory forms and customs declarations switch between naming conventions.
Like many organics, Ethyl 4-Hydroxypiperidine-1-Carboxylate requires gloves, goggles, and good ventilation in handling. Skin or eye contact may cause irritation. Spillage calls for rapid cleanup, since fine powder can get airborne. Storage in tightly closed containers, away from oxidizers and acids, cuts contamination risk. Labs and production plants enforce hands-on safety and routine air monitoring, pushing teams to respect handling procedures, not just file away compliance documentation.
In pharmaceuticals, this compound ranks as a stepping stone toward active ingredients for CNS drugs, pain management compounds, and some antipsychotics. Agrochemical researchers lean on its flexible structure for crafting more selective and environmentally responsible pesticides. The physical chemists and material scientists borrow it to tweak polymer chains or create new surface-active agents. Its real impact shows across fields—if you follow patents, research articles, and regulatory submissions, demand crosses old boundaries, revealing new applications every year.
Every research chemist I’ve worked with wants tools that enable quick iteration. Ethyl 4-Hydroxypiperidine-1-Carboxylate fits because researchers can easily modify the molecule to probe different biological effects or physical properties. Many R&D projects today focus on designing CNS-active molecules where a 4-hydroxy-piperidine base provides the needed “scaffold”. Others explore the ring system for stability improvements, or lower toxicity than precursor chemicals. Academic labs and startups alike embrace it, feeding the compound into screens for bioactivity, optimizing side chains to tip the scales of selectivity or solubility.
Safety profiles drive every application. For Ethyl 4-Hydroxypiperidine-1-Carboxylate, animal studies reveal moderate acute toxicity—high doses trigger drowsiness, lowered motor function, and mild organ effects. Chronic studies remain rare, mainly because end products don’t always include this intermediate. Regulatory dossiers focus on mutagenicity and residual solvent levels, with raw material standards getting tighter every year. Responsible manufacturers commission third-party toxicity screening, a practice that helps everyone downstream: the closer to zero unknowns, the fewer surprises for both workers and patients.
Synthetic chemistry evolves every decade, and intermediates like Ethyl 4-Hydroxypiperidine-1-Carboxylate stand right at the intersection of human health, food production, and new materials. Newer “green” synthesis methods—solvent-free, or using biocatalysts—hold promise for reducing environmental impact, scaling up production safely, and keeping costs reasonable. Drug designers will keep leveraging scaffolds like these as regulatory focus sharpens on molecular safety, not just efficacy. Data-sharing and global safety regulation set high standards, so every gram of intermediate entering the supply chain counts toward safer final products. Innovation in chemical routes and applications rests, in no small part, on keeping foundational compounds like this one available, affordable, and well-characterized.
Ethyl 4-hydroxypiperidine-1-carboxylate might not sound familiar unless you work in pharmaceutical chemistry or spend your days reading medical patents. Yet, this compound helps shape some of the medicines sitting in local pharmacies right now. The reason behind its use is straightforward: it acts as a building block in the early stages of developing complicated drugs, especially those targeting neurological pathways.
For anyone who’s ever watched the methodical pace of drug discovery, it’s always striking to see how seemingly unremarkable chemicals, ones that never end up in the pill bottle, make all the difference behind the scenes. This compound delivers a versatile structure—a piperidine ring. That ring forms the foundation for a whole family of drugs, including antipsychotics, antidepressants, and treatments for Parkinson’s disease.
Drug chemists value ethyl 4-hydroxypiperidine-1-carboxylate for its reactivity. With a little bit of creative chemistry, it can turn into molecules that interact with neurotransmitters in the brain. Take aripiprazole, a well-known antipsychotic, as an example. This sort of drug design relies on tweaking piperidine derivatives, often starting with intermediates like this one. By adding or swapping out side chains, chemists can dial in the drug’s activity and safety.
No one wants to hear about sloppiness in anything health-related, and that goes double for chemicals destined to become medicines. Poor quality at the raw material stage leads to real trouble later. Contaminants, even in tiny amounts, carry through to finished drugs and can cause harmful effects. Pharma companies aren’t rolling the dice with quality. They demand these starting materials meet tough specifications. Third-party audits and traceability for every barrel of chemical boosts transparency and keeps low-quality suppliers out.
Workers handling ethyl 4-hydroxypiperidine-1-carboxylate don’t take shortcuts. Proper gloves, ventilation, and training stand as everyday requirements. Chemical leaks or disposal mistakes cost companies money and reputation, and more importantly, damage the health of real people. Regulators don’t hesitate to punish sloppy practices. Responsible companies adopt green chemistry principles, looking for lower-impact ways to produce not just the medicine, but every single intermediate step. Responsible handling and disposal practices keep contaminants out of water and soil, giving surrounding communities peace of mind.
Chemistry gives modern medicine its backbone. Intermediates like ethyl 4-hydroxypiperidine-1-carboxylate may not grab headlines, but they keep the drug development pipeline flowing. Without them, research teams struggle to make new molecules for clinical trials. Producers of these chemicals have a real duty to invest in quality control, safety, and sustainability. Companies open to better manufacturing techniques, new purification technologies, and alternative raw materials not only make safer products, but also cut down on waste and energy use.
Ethyl 4-hydroxypiperidine-1-carboxylate may only be a small part of a much bigger process, but its importance touches the health of millions. Every improvement at this stage has a ripple effect, leading to safer, more affordable drugs down the road.
Ethyl 4-hydroxypiperidine-1-carboxylate catches more than just the attention of chemists. Sitting at the crossroads between synthetic usefulness and molecular curiosity, its structure explains why the pharmaceutical and research communities pay it so much mind. The core of the molecule, a piperidine ring, links back to substances found in everything from antihistamines to certain antidepressants. Seeing this structure on a chemical drawing reminds me that some patterns in chemistry keep popping up where big changes happen.
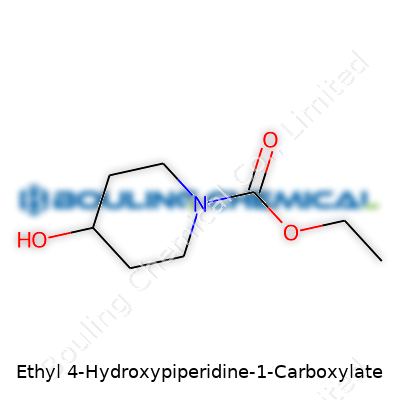
Let’s lay this out—no jargon fences here. At the center, you’ll find a six-membered ring, built from five carbon atoms and a nitrogen. That’s the piperidine ring, which often forms the backbone of dependable, sturdy drugs. Add a twist: at the four position, a hydroxyl group jumps in, which is just an -OH. This changes things. It adds a handle for hydrogen bonds and offers a site for further chemical changes, which chemists use like mechanics reaching for a wrench.
Moving to the beginning, the nitrogen at position one grabs a carboxylate group that’s not floating alone. Attach an ethyl group and the carboxylate suddenly isn’t as acidic, swinging the compound toward moderate solubility in organic solvents and water. Sketch it out, and what you get is:
Breaking down molecules this way always reminds me of my first college synthesis lab. There’s always that moment where you realize the structure isn’t some abstract puzzle. It’s a map for what this molecule can do, where it can go, and how it will behave in a flask or, eventually, in a body. Each part of the structure matters. For example, the hydroxyl can turn this molecule towards water-loving pathways, or support further modeling into drug candidates aimed at specific receptors.
The ethyl ester piece sets up possibilities for cleavage—either through classic acid or base hydrolysis, or through specific enzymes in a living system. This can help tailor a drug’s timing, or help chemists tweak the structure during early synthesis steps. Piperidine’s popularity in drug chemistry only adds weight to this. From antipsychotics to painkillers, many valuable drugs carry a similar ring system, with different functional groups guiding the molecule’s journey through human biochemistry.
Consistent data-sharing and public access to structure drawings would improve the way scientists collaborate and the way non-specialists understand chemical risks and benefits. Anyone interested in the way these molecules shape modern therapy should ask for plain explanations, clear diagrams, and proof from peer-reviewed research. Following standards like those from IUPAC and reporting sources as major science journals do helps guard against mistakes and misleading claims. This holds special weight now, with more people talking about molecules like ethyl 4-hydroxypiperidine-1-carboxylate in the news, but missing context or spreading misinformation.
As I’ve learned in both lab work and writing, structure tells more than a thousand words ever could. Giving it context and connecting it with real-world impact forms the roots of responsible chemical communication. That’s the ultimate foundation of trust between science and everyday life.
Lab work teaches you a lot about the fragility of chemicals. A few stray degrees on a thermometer or a dusty shelf can erase weeks of effort. Ethyl 4-Hydroxypiperidine-1-Carboxylate stands as a reminder that attention to detail matters if purity and safety mean anything in your workflow. Let’s break away from jargon and focus on what storage of this compound truly requires if you want to keep it viable.
Leaving this compound on an open bench or in a random cabinet spells trouble. It should sit in a cool, protected spot—most labs trust a dry, airtight container at room temperature, away from direct light. From first-hand experience, chemical stability slips fast under heat and sunlight, sometimes producing colored impurities or a faint odor shift. Once that change happens, there’s no rolling back the clock.
Moistures does no favor here, either. Ethyl 4-Hydroxypiperidine-1-Carboxylate reacts to humidity with stubbornness. If you scoop from a container that’s picked up some dampness—even just enough to cause clumping—your next synthesis could fail for reasons that don’t show up until it’s too late. So, desiccators and silica packs stop those headaches before they start. Labs that control humidity to under 60% see fewer headaches and re-dos.
Glass vials with tight-fitting lids outrun plastics, especially over several months. Metal containers bring risks of contamination. Clear labeling keeps you from mixing it up with similar, less sensitive compounds, since even trace cross-contamination can shut down a lot of downstream applications. The plain truth: a ten-cent label today saves thousands in repeating botched work.
I once watched a careless coworker open a container in a rush, scattering a fine mist that nobody saw—until someone’s eyes started to burn. Ethyl 4-Hydroxypiperidine-1-Carboxylate won’t explode or catch fire at room temperature, but breathing in dust should not happen. Working in a well-ventilated space, or a fume hood if there's any question, takes a few seconds but blocks a lot of hassle. Wearing gloves and goggles isn't a waste of time; it’s a baseline.
Studies in chemical journals back up personal experience. The National Center for Biotechnology Information lists this compound as stable under dry, room-temperature conditions for long-term storage. It degrades with direct UV exposure and isn't friendly to open-air containers after several days. Data sheets recommend prompt recapping after use. Across reputable chemical suppliers, the emphasis stays on desiccants, dark glass, and minimizing oxygen contact.
You can buy the fanciest storage gear, but care still matters more. Diligence in recapping vials and keeping workspaces clean trumps automation for preventing contamination or spoilage. Routine checks for odor, consistency, or crystalization help you catch early signs of trouble. There’s no substitute for opening your storage drawer and knowing exactly what’s what—because your next reaction depends on it.
Paying attention up front almost always beats cleaning up a mess on the other side.
Ethyl 4-hydroxypiperidine-1-carboxylate has drawn attention in both academic and industrial circles. Chemists use it to build more advanced piperidine compounds, and its similarity to structures in pharmaceuticals and organic synthesis keeps demand steady. The question of bulk access comes up often. Instead of loose talk, people want certainty: Can you really order drums of this chemical from honest-to-goodness suppliers, or are requests met with “inquire for details” and “lead times” that stretch out into months?
On paper, a lot of catalogs show this molecule. The small quantities flow through research supply houses—the kind that focus on 5 or 10 grams at a time. For bulk needs, most find themselves running into a wall. Large, industrial-ready quantities often require placing a custom synthesis order. This usually takes weeks, sometimes longer, depending on the region, regulatory status, and whether the supplier has previously made the compound. The need for regulatory paperwork increases whenever a compound sits close to a controlled substance or resembles structures watched by authorities.
Anecdotally, research teams I’ve worked with have faced wild price swings and unpredictable supplier responses. An order of 1 kg from one supplier in Europe sailed through in three weeks, but a similar attempt six months later turned up empty—down to “raw material shortage.” Reliable, consistent access remains the dream. Despite a surge in specialty chemical companies claiming global reach, actual warehousing of rare building blocks such as this one remains limited. Most companies take orders only after securing a certain minimum quantity from upstream manufacturers—often based in China or India.
For a company scaling up a process or preparing for clinical development, being able to trust that raw materials will be in stock makes or breaks timelines. Delays cost real money. If you can’t source ethyl 4-hydroxypiperidine-1-carboxylate today, the synthetic route chosen last year quickly becomes a liability. For early-stage pharma, the security of supply shapes which molecules get advanced. In my own work, a cheaper or faster supplier always seemed to appear until the day a particular intermediate fell out of stock mid-campaign, wiping out months of planning.
Not all bulk is created equal. Purity among lab-scale samples can seem fine, but on scaling up, impurities creep in—sometimes changing reaction outcomes or failing corporate quality checks. Some brokers cut corners to bring in a product faster, leaving others to clean up the mistakes. Instead of a simple “yes or no” on availability, I always push for the full picture: certification, lot analysis, and references from past buyers.
Partnerships have helped some companies. Ordering through a well-connected chemical distributor with a proven back channel into factories improves odds, even if it costs more. Pooling demand—a trick used by cooperative networks of biotech companies—occasionally secures better deals from manufacturers wary of one-off, unpredictable customers. The rise of blockchain and digital marketplaces promises transparency, but as of now, most buyers still rely on old-fashioned phone calls, direct relationships, and a level of trust.
Before planning that scale-up, confirm bulk supplies directly with manufacturers, not just distributors. Ask for documentation, recent production runs, and be frank about your quantity and timeline needs. Have backup suppliers ready and do not let anyone guarantee “always in stock” without proof.
Sourcing ethyl 4-hydroxypiperidine-1-carboxylate in bulk is possible, but the road is full of challenges—demand, supply security, and quality control all play a role. In my experience, vigilance and relationships drive success much more than any online claim of “available in tons.”
Anyone who has worked in a chemistry lab remembers that sinking feeling when a test result comes out fuzzy, and the culprit turns out to be the purity of a single reagent. Ethyl 4-Hydroxypiperidine-1-Carboxylate falls into that basket. This compound finds its way into research projects, pharmaceutical syntheses, and sometimes, specialty materials. Folks counting on this material rarely aim for “good enough”—they need assurances the sample in the bottle matches what’s written on the label.
The specification for purity sets the floor. Most suppliers offer this compound at a purity of at least 98%, checked by high-performance liquid chromatography (HPLC) or gas chromatography (GC). The remaining 2% covers everything from water content to trace solvents and unseen side products. Researchers often run an NMR or another confirming analysis, especially if a project leaves no room for doubt.
Experience in the lab shows even a one percent difference can make or break a synthesis—especially in pharmaceutical work. A 98% pure sample, in practical terms, leaves roughly ten milligrams of unknowns in a gram. When scaling up or seeking regulatory approval, each milligram counts. Sometimes, people order 99% or higher grades for key steps, or even request analytical data like HPLC traces, to make sure there are no odd impurities lingering in the shadows.
Contamination happens more easily than one might think. Residual solvents from crystallization, moisture taken up during shipping, or unreacted starting material from the last batch can show up in the final jar. In one job, I found a solvent peak in the proton NMR and traced it back to a cleaning solvent used by the supplier. Purity specs exist because these little surprises can have real consequences.
Some buyers request detailed documentation. This often means a certificate of analysis (COA) listing not just the percentage, but breakdowns of water, residual solvents (like ethanol or dichloromethane), and limit tests for heavy metals. Those working towards drug approval want full transparency and consistency.
Reading the fine print, buyers notice whether the percentage refers to “on dry basis” purity or includes water. That small difference explains why some products clump in the bottle or dissolve slower than expected. A chemist learns to ask about how the number was measured, since HPLC, GC, and titration can each show slightly different results for the same sample.
Trust in a chemical supplier comes down to open data and history of reliable shipments. Companies that invest in quality control—regular calibration of their HPLC equipment, traceable standards, and responsive technical support—attract repeat business. The best partners send copies of chromatography traces and answer questions about stability or exact impurity profiles, supporting everyone’s need for robust, defendable results.
Every step of careful manufacturing, from clean glassware to controlled environments and proper documentation, raises the purity level and keeps impurities predictable. Working with feedback from the labs and staying ready to investigate odd results pushes manufacturers to do better. This gives everyone down the line—from a graduate student to a pharmaceutical company—the peace of mind that comes with knowing exactly what sits in each bottle.
| Names | |
| Preferred IUPAC name | Ethyl 4-hydroxypiperidine-1-carboxylate |
| Other names |
Ethyl 1-carbethoxy-4-piperidinol Ethyl 4-hydroxy-1-piperidinecarboxylate |
| Pronunciation | /ˈiːθɪl fɔːr haɪˈdrɒksi paɪˈpɛrɪdiːn wʌn kɑːrˈbɒksɪleɪt/ |
| Identifiers | |
| CAS Number | 252307-32-1 |
| Beilstein Reference | 1718735 |
| ChEBI | CHEBI:196102 |
| ChEMBL | CHEMBL3186583 |
| ChemSpider | 23632645 |
| DrugBank | DB08369 |
| ECHA InfoCard | 03aabe9e-f9d2-486a-89d0-6b3795b6be5f |
| EC Number | EC 628-080-0 |
| Gmelin Reference | 77718 |
| KEGG | C18670 |
| MeSH | D02.241.081.198.250 |
| PubChem CID | 120201242 |
| RTECS number | UU3510000 |
| UNII | IRR8C822MX |
| UN number | UN2811 |
| Properties | |
| Chemical formula | C8H15NO3 |
| Molar mass | 173.23 g/mol |
| Appearance | White solid |
| Odor | Odorless |
| Density | 1.110 g/cm3 |
| Solubility in water | Soluble in water |
| log P | 0.02 |
| Vapor pressure | 0.0005 mmHg at 25°C |
| Acidity (pKa) | pKa ≈ 9.7 (for the 4-hydroxy group) |
| Basicity (pKb) | pKb = 3.38 |
| Refractive index (nD) | 1.498 |
| Viscosity | 106.0 mPa.s (Predicted) |
| Dipole moment | 3.05 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 343.7 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | N04BX19 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes serious eye irritation, may cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS05 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P264, P270, P261, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | Flash point: 143°C |
| NIOSH | No data |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 mg |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
4-Hydroxypiperidine Piperidine-1-carboxylate Ethyl piperidine-1-carboxylate 4-Piperidone 1-Ethoxycarbonyl-4-hydroxypiperidine |